Anthropomorphic pancreas phantom with real-time breathing motion for Carbon-Ion radiotherapy
Christina Stengl,
Germany
OC-0770
Abstract
Anthropomorphic pancreas phantom with real-time breathing motion for Carbon-Ion radiotherapy
Authors: Christina Stengl1,2,3, Ivan D. Munoz4,1,3, Kathrin Panow1, Eric Arbes4,5, Alexander Neuholz6, Stephan Brons6, Armin Runz1,3, Gernot Echner1,3, Jakob Liermann6,7, Oliver Jäkel1,3,6
1German Cancer Research Center, Medical Physics in Radiation Oncology, Heidelberg, Germany; 2University of Heidelberg, Faculty of Medicine, Heidelberg, Germany; 3National Center for Radiation Research in Oncology (NCRO), Heidelberg Institute for Radiation Oncology (HIRO), Heidelberg, Germany; 4University of Heidelberg, Department for Physics and Astronomy, Heidelberg, Germany; 5German Cancer Research Center, Biomedical Physics in Radiation Oncology, Heidelberg, Germany; 6Heidelberg University Hospital, Heidelberg Ion Beam Therapy Center (HIT), Heidelberg, Germany; 7Heidelberg University Hospital, Department of Radiation Oncology, Heidelberg, Germany
Show Affiliations
Hide Affiliations
Purpose or Objective
Pancreatic cancer is the 7th leading cause of cancer related death and its five-year survival rate is only 9% [1]. Treatment options are scarce and the best curable chance is by resection. However, pancreatic cancer patients are often diagnosed in an advanced state, suffering from unresectable cancer. Therefore, novel therapeutic methods are required to improve survival rates. Recently, carbon-ion radiotherapy revealed a favorable oncological outcome [2]. However, results regarding this newly evolving technique are contradicting and require further analysis [3]. For this, we developed an anthropomorphic pancreas phantom with real-time breathing motion and realistic CT/MRI-contrasts for carbon-ion radiotherapy that allows investigating motion induced uncertainties of the delivered dose.
Material and Methods
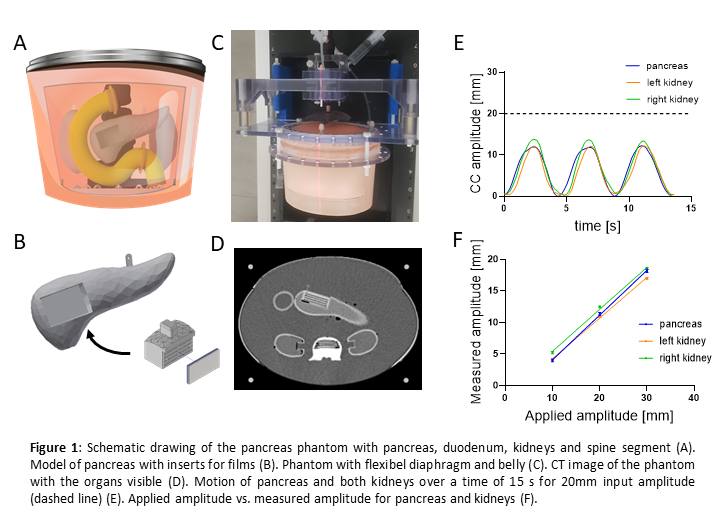
The proposed pancreas phantom resembling the abdomen, contains a deformable diaphragm and peritoneum, a pancreas, two kidneys, a duodenum and a spine segment (Fig. 1 A-D). 3D printed pancreas and kidney models were prepared with NiDTPA-KCl-agarose mix [4] to achieve human-equivalent CT and MRI contrasts. The duodenum was 3D printed with elastic material to enable morphological changes during emptying. Breathing motion with an amplitude of 10mm, 20mm and 30mm was applied by means of a computer-controlled hydraulic system to quantify organ motion. Carbon-ion irradiation was realized with a 4Gy (RBE) single fraction irradiation schedule to resemble standard patient plan geometry. For dosimetric measurements, a PinPoint ionization chamber and EBT3 films were inserted in the organs via an easy plug-in mechanism. Thereafter, breathing motion with 30mm amplitude was compared with static setup during irradiation.
Results
Our pancreas phantom presented reproducible motion for the breathing patterns with increasing amplitude. The pancreas motion revealed (3.98±0.36)mm, (11.38±0.41)mm and (18.19±0.44)mm for 10mm, 20mm and 30mm input amplitude, respectively, presenting a linear fit with an R² of 0.996 (Fig. 1 E, F). Therewith, any motion amplitude can be calculated and applied according to individual patient pancreas motion. By clearing the duodenum, its volume decreased by 37%. Measurements with the ionization chamber revealed dose deviations of 15% during pancreas motion, which was significantly higher than in static case (0.26%). Also with films, inhomogeneous dose distributions at the target site were observed.
Conclusion
These results demonstrated the suitability of the presented phantom for motion studies in carbon-ion radiotherapy. Easy exchange of dosimeters and adaptable breathing motion makes this phantom feasible to test not only motion management strategies but also combine it with FLASH and Mini-beam irradiation for our future studies to improve pancreas cancer treatment.
References
[1] Rawla et. al., doi: 10.14740/wjon1166
[2] Kawashiro et. al., doi: 10.1016/j.ijrobp.2018.04.057
[3] Liermann et. al., doi: 10.3389/fonc.2021.708884
[4] Elter et. al., doi: 10.1088/1361-6560/abd4b9