Acute toxicity reported by patients with prostate cancer receiving online MR-guided radiotherapy
OC-0136
Abstract
Acute toxicity reported by patients with prostate cancer receiving online MR-guided radiotherapy
Authors: Pia Krause Møller1, Lars Dysager1, Uffe Bernchou2, Anders Smedegaard Bertelsen2, Carsten Brink3, Faisal Mahmood2, Henrik R Jensen2, Olfred Hansen1, Christina J Nyborg1, Helle Pappot4, Karin B Dieperink1
1Odense University Hospital, Department of Oncology, Odense C, Denmark; 2Odense University Hospital, Laboratory of Radiation Physics, Odense C, Denmark; 3Odense University Hospital , Laboratory of Radiation Physics, Odense C, Denmark; 4Rigshospitalet, Department of Oncology, Copenhagen, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
The study Prostate Radiotherapy Integrated
with simultaneous MRI (PRISM-OUH) was initiated to assess feasibility and
tolerability of online adaptive radiotherapy on the 1.5 T MR-linac (oMRgRT) for
patients with localized prostate cancer (intermediate risk). To achieve the
complete picture of tolerability, the aim of the present study was to describe
the development in patient-reported outcomes (PROs) over a six months period
following oMRgRT.
Material and Methods
For patients with prostate cancer, oMRgRT was delivered in 20 fractions
with daily online adaptation. Radiotherapy (RT) was planned to a total dose of 60 Gy in the prostate and proximal 1 cm
of the seminal vesicles (SV), and 48 Gy to an additional 1 cm of the SV. PTV margins were 5 mm isotropic, except for 3
mm posteriorly for PTV 60 Gy. To
assess patient symptoms and HRQoL, the patients reported the International
Prostate Patient Index (I-PSS, lower score=lower symptom burden) and the
Expanded Prostate Cancer Index (EPIC-26, higher score=better HRQoL) at
baseline, end of RT, and one, three and six months after end of RT. In addition,
HRQoL was assessed with the EUROQOL EQ-5D-5L (1=max HRQoL) at the same time
points.
Results
In total, 31 patients were included; median age 69 (46-76), Gleason
score 6 (n=4) and 7 (n=27), 78% had ECOG/WHO performance status 0, and 48%
received six months of LHRH agonists. Out of 620 planned treatment fractions,
26 were delivered on a CT-based linac (15 to one patient finding the treatment
time hard to tolerate, 11 due to technical problems).
Moderate/severe urinary symptoms was reported by 74% of the patients
at the end of RT (highest mean I-PSS score 13.6). This score was still above
the pre-treatment level (6.5) one month following RT (11.3). Three months
following RT, the same proportion of patients with moderate/severe urinary
symptoms as pre-treatment (30%) was obtained (Table 1). These findings were
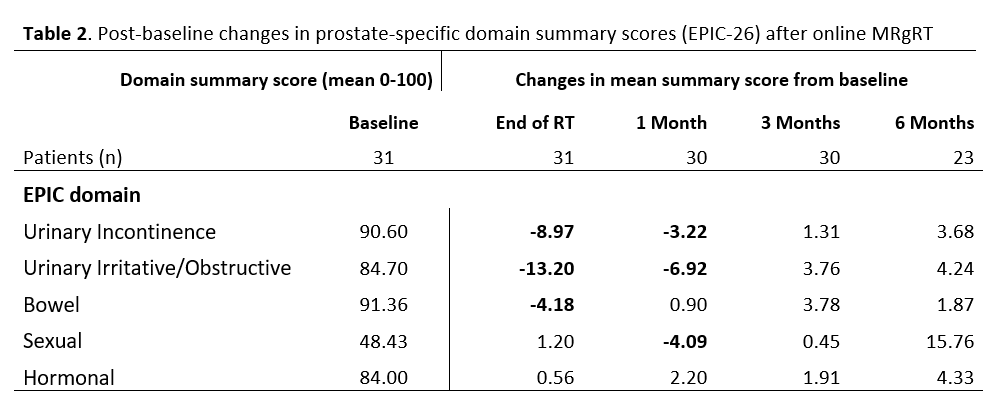
consolidated in the EPIC-26 domain scores. In three domains (urinary
incontinence, urinary irritative /obstructive and sexual), the pre-treatment
summary score was not reached until three months following RT (Table 2). Pain
or burning on urination was one of the symptoms worsened one month after RT
(15% vs 3% baseline).
Patient satisfaction with their urinary condition reached the
pre-treatment level one month after RT (Table 1). The HRQoL of the patients was
stable throughout oMRgRT (EQ index score 0.907) and improved one and three
months following RT (0.911-0.951).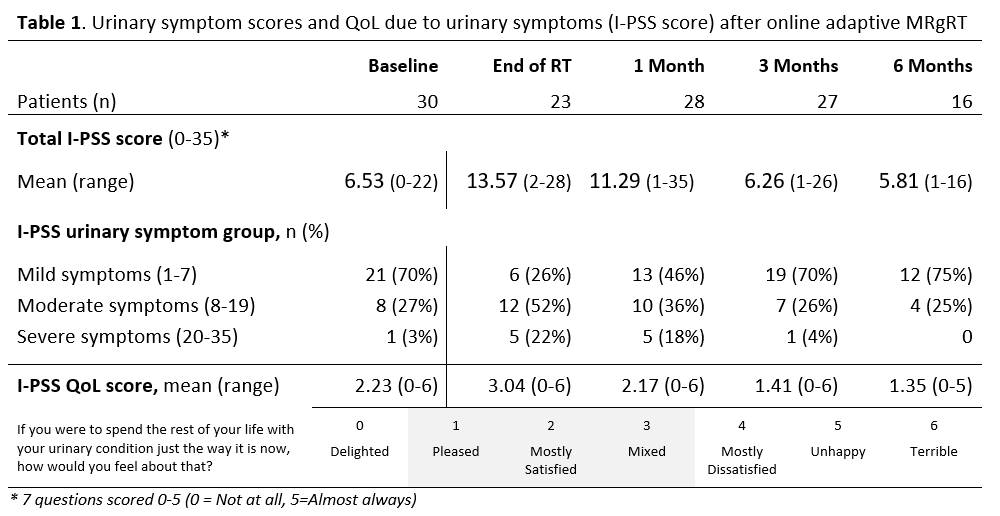
Conclusion
Patients with localized prostate cancer reported
some acute urinary toxicities and sexual problems still being increased one
month following oMRgRT but reduced to the pre-treatment level at three months
following oMRgRT. However, an increased HRQoL was reported after the end of
oMRgRT. The acute symptom trajectory outside the fixed time-points remains to be
investigated, thus a prospective study with weekly PROs is initiated.