Most radiotherapy treatments are fractionated because normal tissues can tolerate higher total doses if the dose is split into multiple fractions. However, more fractions also require higher doses in the tumor to achieve the same level of tumor control. In that regard, the ideal treatment would simultaneously achieve hypofractionation in the tumor along with more uniform fractionation in normal tissues. While this may appear impossible at first glance, it can be partially achieved by delivering distinct dose distributions in different fractions. These dose distributions are designed such that different fractions deliver high single-fraction doses to complementary parts of the tumor while achieving a similar dose bath in the surrounding normal tissue, thereby increasing the biological effect in the tumor while exploiting the fractionation effect in the normal tissue [1,2]. We refer to this concept as spatiotemporal fractionation.
The first part of the presentation will introduce spatiotemporal fractionation and discuss potential clinical applications in the context of in-silico treatment planning studies. We will introduce treatment plan optimization methodology to simultaneously optimize multiple dose distributions for different fractions based on objectives and constraint functions evaluated for the cumulative biologically effective dose (BED) of all fractions. Spatiotemporal fractionation is then demonstrated for patients with multiple brain metastases, illustrating a potential clinical application.
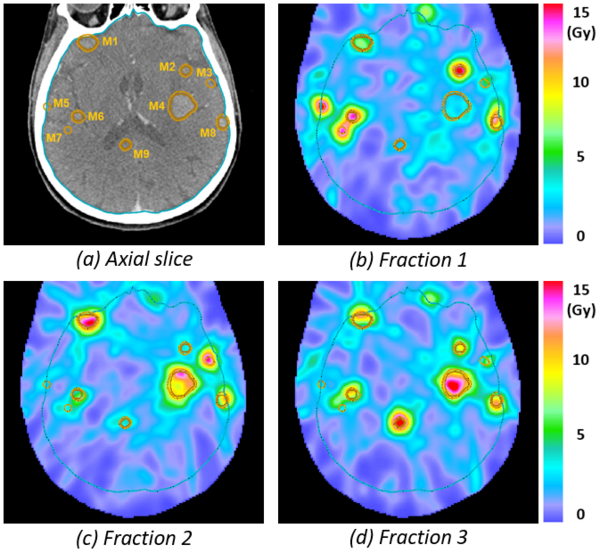
The figure illustrates spatiotemporal fractionation for a patient with 30 brain metastases, out of which 9 are shown on the axial slice. A 3-fraction SRS treatment is shown with a prescription corresponding to the BED10-equivalent of 3x9 Gy or 1x18 Gy. Spatiotemporal fractionation delivers high single fraction doses to small metastases. For example, metastasis M5 receives most of the dose in fraction 1 whereas M9 receives most of the dose in fraction 3. In addition, larger metastases such as M4 are compartmentalized into different regions that receive high doses in alternate fractions. Thereby, the prescribed BED10 can be achieved with a lower physical dose. Since some degree of fractionation is achieved in parts of the normal brain between the metastases, the lower physical dose in the tumor results in a net reduction of the mean BED2 in the normal brain. Compared to a treatment delivering the same dose distribution in all 3 fractions, spatiotemporal fractionation achieves mean brain BED2 reductions of approximately 15%.
The second part of the presentation will present initial work towards a preclinical validation of spatiotemporal fractionation in small animal models. While an end-to-end validation of the concept as a whole is impractical due to technical and biological limitations, individual underlying assumptions may be tested. One of the key assumptions of spatiotemporal fractionation is that different parts of the tumor can be treated to different doses in different fractions, and tumor control is maintained as long as the prescribed cumulative BED is delivered to all parts of the tumor by the end of the treatment. Ongoing work tests this assumption in subcutaneous tumors in mice using a high-precision image-guided small animal radiotherapy research platform [3].
[1] J. Unkelbach, M. Bussière, P. Chapman, J. Loeffler, H. Shih. Spatiotemporal Fractionation Schemes for Irradiating Large Cerebral Arteriovenous Malformations. Int. J. Rad. Onc. Biol. Phys., 2016;95(3):1067-1074
[2] J. Unkelbach, D. Papp, M. Gaddy, N. Andratschke, T. Hong, M. Guckenberger. Spatiotemporal fractionation schemes for liver stereotactic body radiotherapy. Radiother. Oncol. 125(2):357-364, 2017
[3] I. Telarovic, J. Krayenbuehl, I. Grgic, F. Tschanz, M. Guckenberger, M. Pruschy, J. Unkelbach. Probing spatiotemporal fractionation on the preclinical level. Phys. Med. Biol. 65(22):22NT02, 2020