Patient recruitment by RTTs yields high accrual in a randomized trial of palliative radiotherapy.
Ann Christin Lund,
Denmark
PO-1858
Abstract
Patient recruitment by RTTs yields high accrual in a randomized trial of palliative radiotherapy.
Authors: Ann Christin Lund1, Anna Mann Nielsen2, Aleksandra Caranovic3, Jeppe Friborg1, Vibeke Nordmark Hansen1, Tanja Stagaard Johansen1, Bente Sommer Kristensen1, Romain Marseguerra1, Kamilla Nor Matthiesen1, Lisbet Prenter1, Deborah Anne Schut1, Sanne E. Tvile1, Anja Munk1, Dorthe Sørensen1, Anna Green1, Marie Langballe4, Tina Rantzau Bratbjerg Jensen1, Anne Frank Fastø1, Louise Aalund1, Gitte Fredberg Persson5, Ivan R. Vogelius1, Morten Hiul Suppli1
1Rigshospitalet, University of Copenhagen, Dept. of Oncology, Copenhagen, Denmark; 2Herlev-Gentofte Hospital, University of Copenhagen, Herlev, Dept. of Oncology, Herlev, Denmark; 3Rigshospitalet, University of Copenhagen , Dept. of Oncology, Copenhagen, Denmark; 4Rigshospitalet, University of Copenhagen, Dept. of Oncology, Copenhagen,, Denmark; 5Herlev-Gentofte Hospital, University of Copenhagen, Dept. Of Oncology, Herlev, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
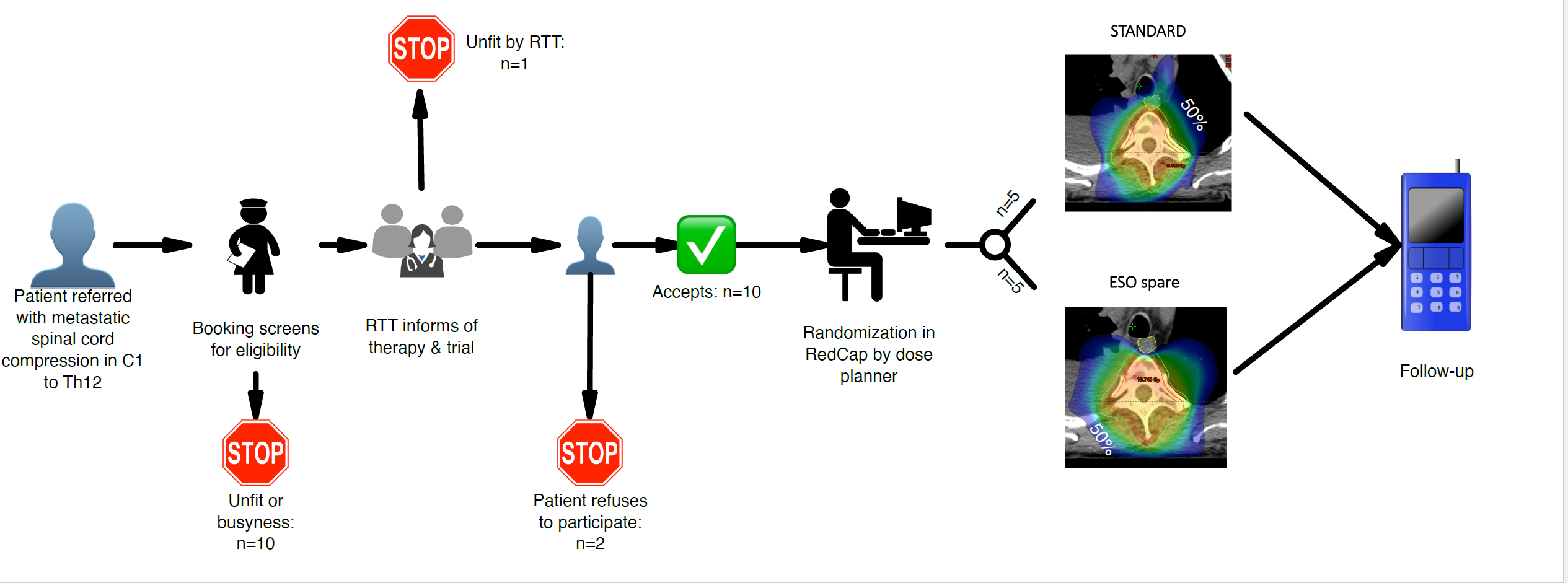
The ESO-SPARE randomized trial compares
esophagus-sparing radiotherapy to standard IMRT for palliation of metastatic spinal
cord compression. See Figure 1 for trial dose planning examples. Primary
endpoints are patient reported gastro-esophageal toxicity and preservation of
ability to walk. Sample size is 2x100 patients to be accrued in two hospitals.
We aim to facilitate a high accrual rate through integration with the clinical
functions in a pragmatic trial setup driven by RTTs.
Material and Methods
The ESO-SPARE trial team consists of 7 RTTs in
rotation that screen for eligibility, provide trial information and acquires
patient consent as integrated part of clinical routine. The team is equipped
with a designated “trial phone” for easy access. A patient information kit and material
for follow-up are provided to the patient by the same staff.
After consent, the RTT register the patients
baseline data in Redcap. The dose planning RTTs complete randomization immediately
prior to generating the dose plan pertaining to the trial arm selected by the
randomization procedure. A radiation oncologist approves all dose plans.
A screening log of all patients with metastatic
spinal cord compression between C1 and Th12 vertebrae was planned.
Regular meetings to optimize the workflow are
being held and here we report the first three weeks of accrual in a single
center (trial opened Oct 4th, 2021).
Results
4 patients were accrued in the first week, 3 in
the second and 3 in the third week (holiday week). Staff shortage caused the
accrual to be closed for 4 out of 15 days. Still accrual was three times higher
than the design-anticipated 1 patient per week.
All
but one patient (referred for surgery before RT) on trial started treatment
within two weekdays days from referral. Maintaining
the originally planned screening log resulted in a too high burden of
registration, so the procedure has been updated to only maintain screening logs
for patients addressed by the ESO-SPARE trial team.
Conclusion
The RTT driven patient recruitment for a RCT is
feasible and highly efficient. The first three weeks of accrual suggest a
higher than expected accrual rate at a comparatively modest resource
expenditure. We propose that involving RTTs in patient accrual and running
clinical trials is a viable and promising approach to increase the speed of
knowledge generation in radiation oncology.