Clinical outcomes after interstitial HDR brachytherapy for locally advanced cervical cancer
Paula Vicente Ruiz,
Spain
PO-1793
Abstract
Clinical outcomes after interstitial HDR brachytherapy for locally advanced cervical cancer
Authors: Paula Vicente Ruiz1, Beatriz Vazquez Barreiro1, Lucia Rodriguez Ruiz1, Jose Luis Guinot Rodriguez1, Maribel Tortajada Azcutia1, Miguel Angel Santos Olias1, Victor Gonzalez Perez2, Alonso de la Rosa de los Rios1, Leoncio Arribas Alpuente1
1Fundación Instituto Valenciano de Oncología, Radiation Oncology, Valencia, Spain; 2Fundación Instituto Valenciano de Oncología, Radiophysics, Valencia, Spain
Show Affiliations
Hide Affiliations
Purpose or Objective
Brachytherapy (BT) is mainstay in the standard of care for patients receiving primary curative-intent for locally advanced cervical cancer because it is the best technique to deliver significantly higher doses to the target while minimizing dose to the normal tissues. Interstitial BT allows for better dosimetry and higher doses. We analyze Progression-free survival (PFS) in terms of local, regional (defined as pelvic relapse) and distant relapse rate, time to local progression, overall survival (OS) and toxicity.
Material and Methods
We retrospectively evaluated patients with locally advanced cancer stage IB-IVB (FIGO Staging System 2018) of the cervix who completed treatment with EBRT to a total dose of 50 Gy (encompassing primary tumor and regional pelvic lymphatic nodes) with concurrent cisplatin-based chemotherapy and Ir-192 endocavitary and interstitial BT. It was guided by computed tomography or magnetic resonance image. Dose is prescribed to the HR-CTV (including cervix and parametrial involvement). We recorded total dose to the isodose D90 of the HR-CTV, maximum dose to 2 cc bladder, rectum and sigma (DV2cc, DR2cc and DS2cc). Toxicity was reported according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
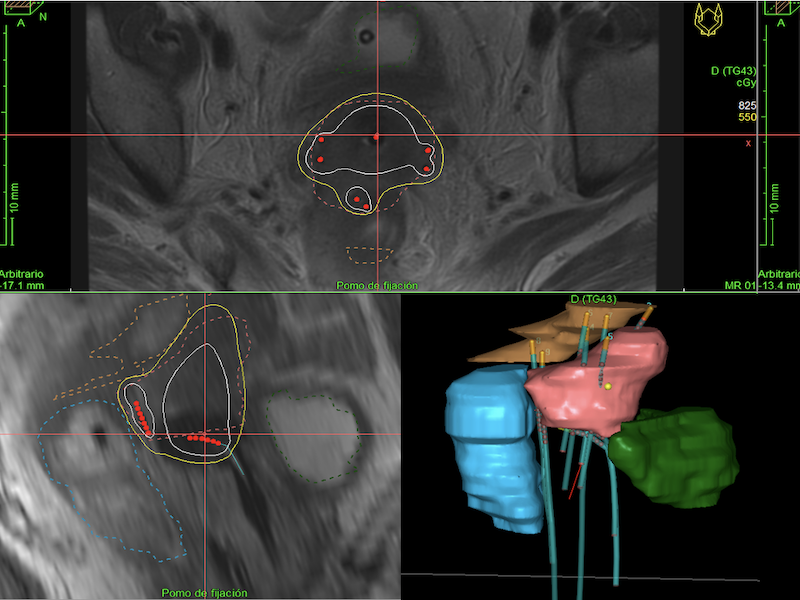
Results
We recruited 35 women with primary squamous cell carcinoma (n=30), adenocarcinoma (n=4), and neuroendocrine (n=1) carcinoma of the cervix who have been treated between 2015 and 2020. The median age at diagnosis was 49 years (25-71 years). Eight (22.8%) patients underwent paraaortical lymphadenectomy and 12 (34.3%) patients received EBRT to paraaortics. Twenty-one (60%) patients had concomitant boost to affected lymph nodes. Total mean dose of both EBRT and BT was EQD2 86.3 ± 2.3 Gy, completed in an average of time of 59 days. After a median follow-up of 34.5 months (6-74 months), 28 women are alive and 23 remain disease-free. The OS was 84.9% at 24 months, and 80.2% at 36 months. Only 4 women presented local relapse and it occurred before 15 months. Eleven patients (31.43%) had extrapelvic metastases in bone (n=2), lung (n=2), liver (n=1), CNS (n=2), and distant lymph nodes (n=8). The maximum EQD2 to the 2 cc of bladder, rectum, and sigma was 75.4 ± 6.6 Gy, 66.1 ± 7.4 Gy, 65 ± 12.6 Gy respectively (mean ± standard deviation). No grade≥3 urinary, gynecological or rectal toxicities have been observed to date. Three patients presented grade 2 rectovaginal fistula and one patient presented grade 1 proctitis. Every registered death (n=7) was due to disease progression.
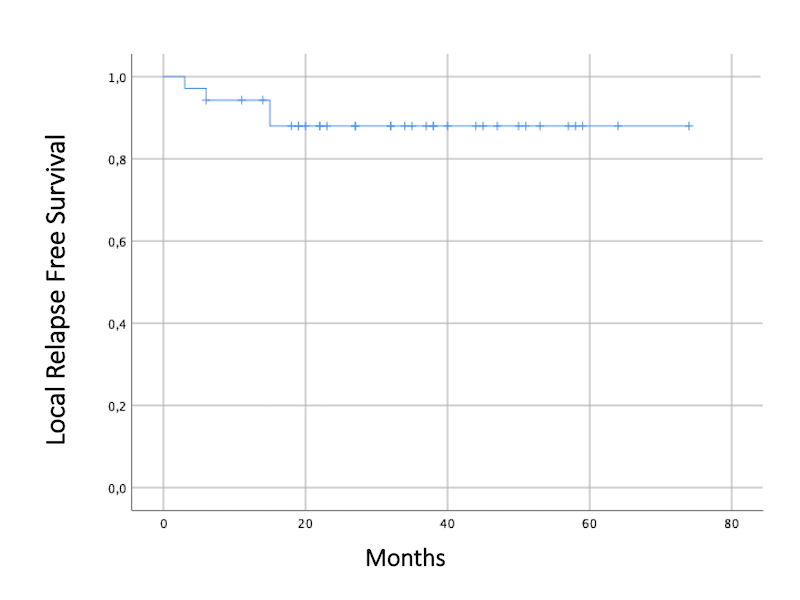
Conclusion
BT is essential and effective for curative cervical cancer treatment, delivering high dose to the HR-CTV with maximum sparing of OARs using interstitial needles. We achieve a great local control (88% at 36 months) and OS (84.9% at 24 months) with low rates of severe toxicity for women with locally advanced disease. Therefore, we recommend the use of interstitial BT whenever possible.