Automated treatment planning using dose mimicking for biologically guided dose prescription
PO-1734
Abstract
Automated treatment planning using dose mimicking for biologically guided dose prescription
Authors: Ana Ureba1,2, Jakob Öden3, Iuliana Toma-Dasu1,4, Marta Lazzeroni1,4
1Stockholm University, Department of Physics, Stockholm, Sweden; 2Karolinska Institute, Oncology and Pathology department, Solna, Sweden; 3RaySearch Laboratories AB, Research department, Stockholm, Sweden; 4Karolinska Institute, Oncology and Pathology Department, Solna, Sweden
Show Affiliations
Hide Affiliations
Purpose or Objective
This work presents an automated approach for treatment planning using a dose
painting strategy (DP) based on the combined information on the tumour
clonogenic cell number (CCN) and on the tumour oxygen distribution derived from
PET images.
Material and Methods
The treatment planning workflow was created in RayStation (v10,
RaySearchLaboratories) via scripting. The automated treatment plan pipeline
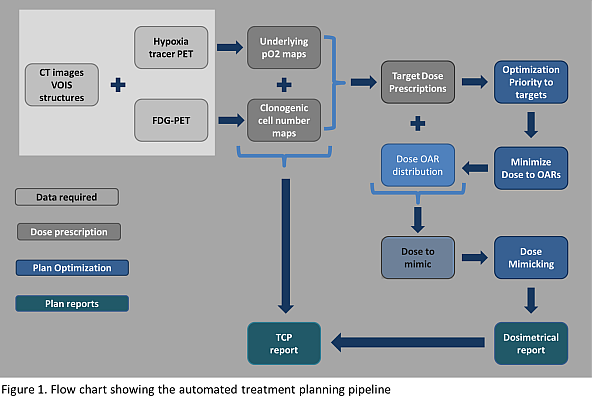
consists of 3 stages (fig. 1):
Dose prescription: Normalized uptake of a PET tracer dedicated to
hypoxia is converted by means of a non-linear function into oxygen partial
pressure maps (pO2). Dose modifying factors to counteract
radioresistance in the hypoxic areas are calculated based on the obtained pO2
values. The CCN is derived from FDG-PET images as follows: the normalized
uptake of FDG is converted into CCN by means of a linear conversion function, whose
curve origin and slope were derived from the patient dataset. The DP strategy aims
at 95% tumor control probability (TCP) in the CTV. Three levels of uniform dose
are assigned to the hypoxic target volume (HTV), to the GTV-HTV and to the CTV-GTV.
Plan optimization: The algebra combination of the volumes of interest
(VOIs) is automatically performed prior to optimization. The whole optimization
process is composed of three different steps: 1) two optimizations where targets
are prioritized; 2) an optimization of previous solution where the dose to organs
at risk (OARs) is reduced to meet the clinical dose constraints; 3) two minimax
robust optimizations that mimicked both the OAR doses retrieved from (2) and
the prescribed doses to the targets.
Plan Evaluation: A dosimetric evaluation of the nominal plan is performed
accounting for target coverage and OAR constraints. The target TCP is calculated
by considering the underlying radiosensitivity and CCN derived from the PET
images in the initial stage.
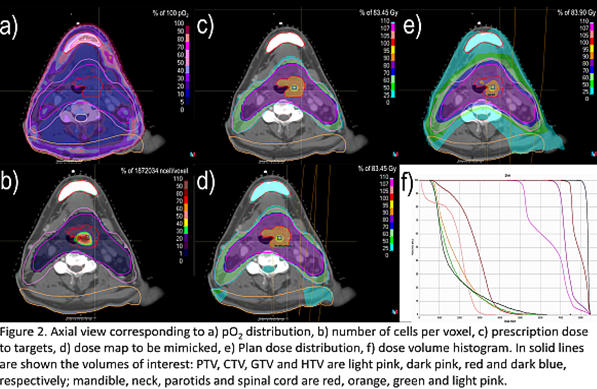
Results
The presented treatment
planning pipeline was tested on a head and neck cancer case imaged with FMISO
as hypoxia tracer. The automated treatment planning pipeline was proven able to
render a dose distribution that successfully met the clinical goals (Fig.2) with
the following plan specifications: the total dose delivered in 35 fractions
with an integrated boost. The minimax robust optimization considering ±3mm
setup errors (7 scenarios) was performed. The whole optimization process
required about 45 minutes to be performed.
Conclusion
The presented automatic treatment planning workflow
has shown to be feasible and it can be readily applied to different treatment
sites and modalities supported by the treatment planning system.