Evaluating the effectiveness of interplay mitigation techniques in proton therapy
Huygen van der Wind,
The Netherlands
PO-1700
Abstract
Evaluating the effectiveness of interplay mitigation techniques in proton therapy
Authors: Huygen van der Wind1, Oscar Pastor-Serrano1, Steven Habraken2,3, Dennis Schaart1,3, Danny Lathouwers1, Mischa Hoogeman2,3, Zoltán Perkó1
1Delft University of Technology, Department of Radiation Science and Technology, Delft, The Netherlands; 2Erasmus MC Cancer Institute, Department of Radiotherapy, Rotterdam, The Netherlands; 3HollandPTC, Department of Radiation Oncology, Delft, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Breathing interplay effects arise from the interaction between the moving tumor and scanning beam. Various mitigation techniques and planning methodologies account for tumor motion during dose delivery, yet a comprehensive comparison of how combinations of these perform is missing. This study aims to investigate the effectiveness of a wide range of interplay mitigation approaches under various fractionation schemes.
Material and Methods
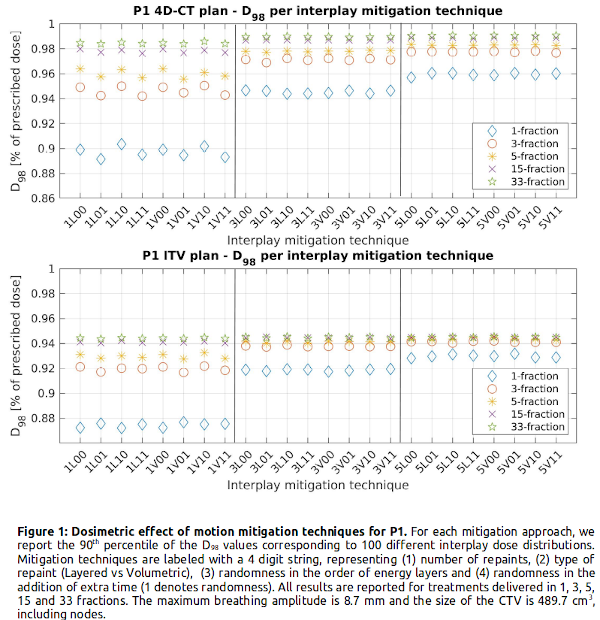
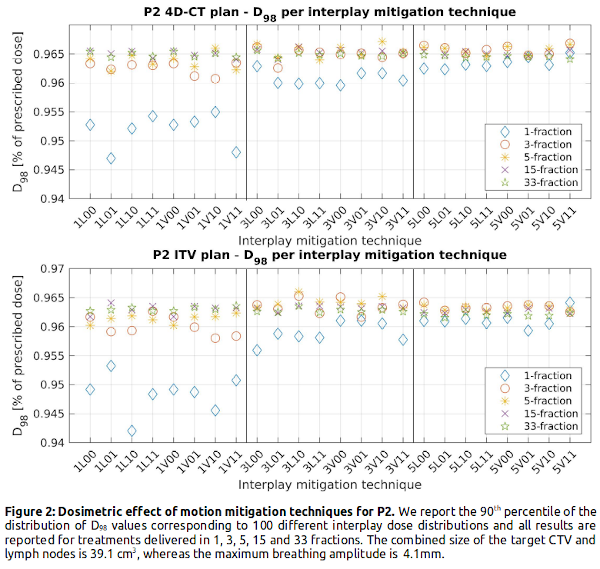
We statistically assess interplay effects in 4D-CT anatomically robust (using 5 phases) and ITV plans (originally planned for 33 fractions of 2 Gy/fraction based on clinical practice at HollandPTC, Netherlands) of two lung cancer patients: P1 with a CTV volume of 489.7 cm3 and maximum breathing amplitude of 8.7 mm, and P2 with a CTV of 39.1 cm3 and 4.1 mm maximum amplitude. The interplay dose calculation is based on 8 phase 4D-CT scans and breathing signals indicating the phase at which each spot is delivered. As motion mitigation techniques we simulated fractionation (with 1, 3, 5, 15 and 33 fractions); layered and volumetric rescanning (with 1, 3, and 5 repaints); random extra time addition between spots; random energy layer ordering; and all possible combinations of these. Using 100 different breathing scenarios (100 different breathing signals) per mitigation setting, our analysis is based on the 90th percentile of the 100 corresponding D98 values, expressed as a fraction of the prescribed dose. Doses for hypofractionated treatments are adjusted to be biologically equivalent to the 33 fraction scenario using biologically effective dose with α/β = 10Gy.
Results
Figure 1 shows that adding more repaints has little added value for >15 fractions, and that treatments delivered in 5 fraction with 5 repaints per fraction are almost equivalent to 33 fraction delivery. With similar values for the same number of repaints, rescanning provides the same level of smoothing regardless of being volumetric, layered or including randomness. Our results also indicate the inherent fragility of ITV, shown by the 4% (P1) and 0.5% (P2) decrease in D98 with respect to 4D-CT robust plans across all mitigation settings. For the small tumor (P2), adding 2 more repaints has no large effect on the D98, whereas for the larger tumor (P1) 5 repaints results in a higher D98 in the hypofractionation regime. For larger tumor volume and movement, the spread in D98 increases between the pure interplay scenario and the interplay mitigated scenarios (P1: 88%-99% vs. P2: 94%-97% of the prescribed dose), indicating that interplay mitigation is more important for larger tumor and motion.
Conclusion
Comparing the D98 of clinical treatment plans across 24 different motion mitigation settings indicates that adding randomness has no effects and that 4D-CT plans are intrinsically more robust compared to ITV plans,. While rescanning is highly beneficial in hypofractionated treatments, fractionation makes rescanning unnecessary in standard fractionated schemes.