Risk management plan for the 3D printed bolus in radiotherapy
PO-1667
Abstract
Risk management plan for the 3D printed bolus in radiotherapy
Authors: MARIA LIZONDO1, Elisabet Gonzalez Lao2, Luis Oraa López3, Branly Parra Prieto3, Carles Fabres Martín3, Toni Ramírez1, David Navarro1, Julia García-Miguel1, Eva Ambroa1, Teresa Valdivielso1, Ángel Infiestas1, Ángel Lorenzo1, Ursula Gallardo1, Annie Peralta1, Gemma Frontera1, Debora Amat1, Ana López1, Sonia Almendros4, Maypi Ballesteros2, Marc Parcerisa1, Ramon Pujol5, Montserrat Colomer5
1Consorci Sanitari de Terrassa, Medical Physics Unit, Radiation Oncology Department, Terrassa, Spain; 2Consorci Sanitari de Terrassa, Quality and Patient Safety Department, Terrassa, Spain; 3Consorci Sanitari de Terrassa, Orthopedics and Traumatology Department, Terrassa, Spain; 4Consorci Sanitari de Terrassa, Radiation Oncology Department, Terrassa, Spain; 5Consorci Sanitari de Terrassa, Medical Physics Unit, Radiation Oncology , Terrassa, Spain
Show Affiliations
Hide Affiliations
Purpose or Objective
The introduction of 3D printing in health has opened our minds to a
world of endless possibilities. One of these is the use of personalized 3D
printed bolus for their use in radiotherapy, to adapt perfectly to the surface
of the patient during all treatment sessions. Its superiority over other
commercial bolus has been largely tested.
Customized medical devices are contemplated in the new Regulation (EU)
2017/745, requiring a risk analysis before their manufacture, to avoid any
adverse event that may affect clinical safety.
The purpose of this study is to analyze the possible risks in the 3D
printed bolus, establish regulatory requirements, and ensure the safety of our
patients.
Material and Methods
The process for the bolus production is the following. First, the bolus
design is made in Monaco (v.5.51) based on the anatomy of the patient. After
that, the file structure created is exported and converted to a .stl file with
3DSlicer (v.4.11). Before it is ready to print, smoothing is needed (Meshmixer,
v.3.5). Finally, the file is sent to Ultimaker Cura (v.4.6.1) printer and
printed with PLA filament. As this bolus is personalized, it is used by only
one patient during all the treatment following medical considerations.
A multidisciplinary working group composed of a Radiation Oncologist, Medical
Physicists, 3D-printing experts, and the person in charge of regulatory in
medical devices from our institution analyzed the risks associated with every
stage of the bolus production and its application according to ISO 14971:2019 “Medical
devices - Application of risk management to medical devices”. Actions required
to minimize the risks were also elaborated.
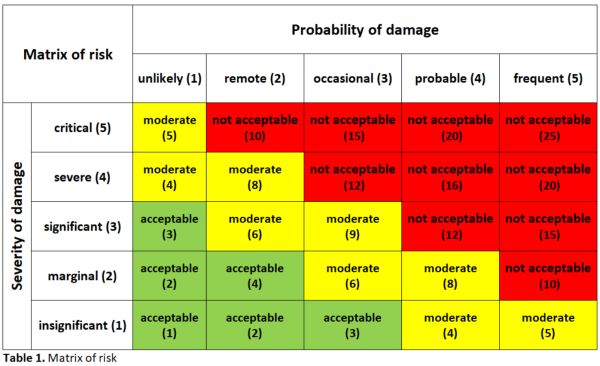
A risk-impact matrix was used to identify potential risks. It provides
the impact of the medical devices on patient safety (the probability of
occurrence of harm and the consequences of that harm). The multiplication of
these two factors gives us a risk priority index (RPI) to establish preventive,
corrective, or improvement actions before its use in patients (Table 1).
Results
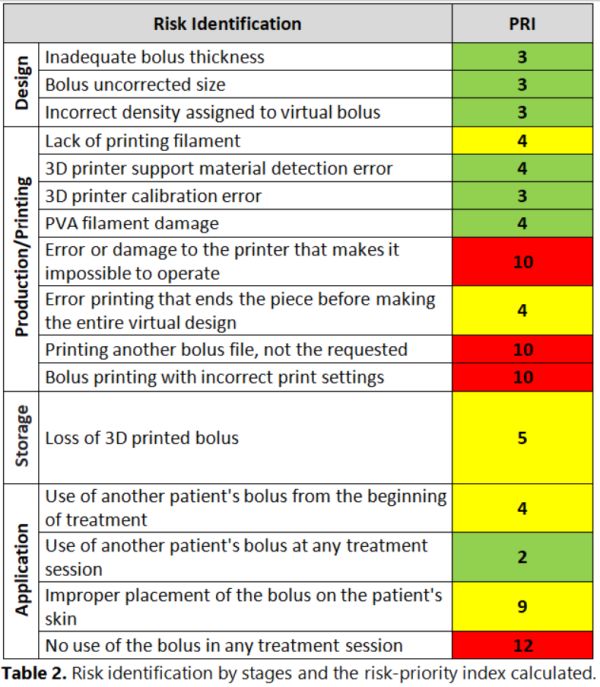
The working group identified 4 stages (Design, Production/Printing,
Storage, and Application) and 16 risks in the 3D printing process of radiation
therapy boluses, which were analyzed and evaluated (Table 2). The risks were
distributed as follows: 3 in the design process, 8 in 3D printing, 1 in storage,
and 4 in the application. The critical risks were considered with PRI>5 and
special attention was paid to elaborate actions to minimize them. For example, an image registration between the virtual bolus and a CT of
the printed bolus is proposed for the risk “Printing
another bolus file, not the requested”.
Conclusion
We elaborated analysis of the risk factors present in the 3D printed
bolus production as well as proposed actions to stop or minimize them. This can
be applied to any Radiotherapy and Medical Physics department. Its use inside a
management programme will reduce the risks of using this medical device, and
its impact for the patient safety.