Reproducibility of quantitative PET in radiotherapy setup for multicenter PET/MR in head/neck cancer
PO-1653
Abstract
Reproducibility of quantitative PET in radiotherapy setup for multicenter PET/MR in head/neck cancer
Authors: René Mario Winter1, Ola Engelsen2, Oddbjørn Sæther3, Kathrine Røe Redalen4
1Norwegian University of Science Technology, Department of Physics, Trondheim, Norway; 2University Hospital of North Norway (UNN), PET Imaging Center, Tromsø, Norway; 3St. Olavs hospital, Trondheim University Hospital, Department of Radiology and Nuclear Medicine, Trondheim, Norway; 4Norwegian University of Science and Technology, Department of Physics, Trondheim, Norway
Show Affiliations
Hide Affiliations
Purpose or Objective
18F-fluorodeoxyglucose (FDG)-PET is one of the most investigated functional imaging techniques in multicenter trials for radiotherapy. The use of different imaging systems in a multicenter trial, however, has the disadvantage that it may add variation to the quantitative image values. This is of particular concern in combined PET/MR systems tailored to imaging the patient in radiotherapy position, since these require PET correction methods with custom made attenuation maps of the hardware in the PET image reconstruction. Variation in the implementation of the custom maps may increase the variation in PET image values. Our aim was to investigate the bias and reproducibility of quantitative PET on a radiotherapy tailored PET/MR system in the participating centers of a multicenter imaging trial in head/neck cancer (EMINENCE, clinicaltrials.gov NCT04612075).
Material and Methods
Performance of PET was assessed on a 3T PET/MR system (Siemens) with radiotherapy setup for head/neck (Medibord; Fig1A) in two centers. The radiotherapy setup consisted of a flat tabletop, coil holders and body coils. FDG-PET was measured in a homogeneous phantom (2L bottle with FDG in water). PET scans of the phantom were performed repeatedly with mounted and unmounted radiotherapy setup. Image reconstructions were performed with a CT-based attenuation map of the radiotherapy hardware setup and without to determine the relative bias in custom attenuation corrected PET. For comparison, PET data acquired with radiotherapy setup was reconstructed without the custom map to determine the bias of uncorrected PET. The reference scan without radiotherapy setup was repeated two more times to determine the error of repeating scans and after repositioning the phantom. All PET images were decay corrected to the start time of the first scan. In all image reconstructions, the same DIXON MRAC attenuation map of the phantom was used. FDG-PET standardized uptake value (SUV) correction with the custom map was tested in the first patient recruited in the EMINENCE trial.
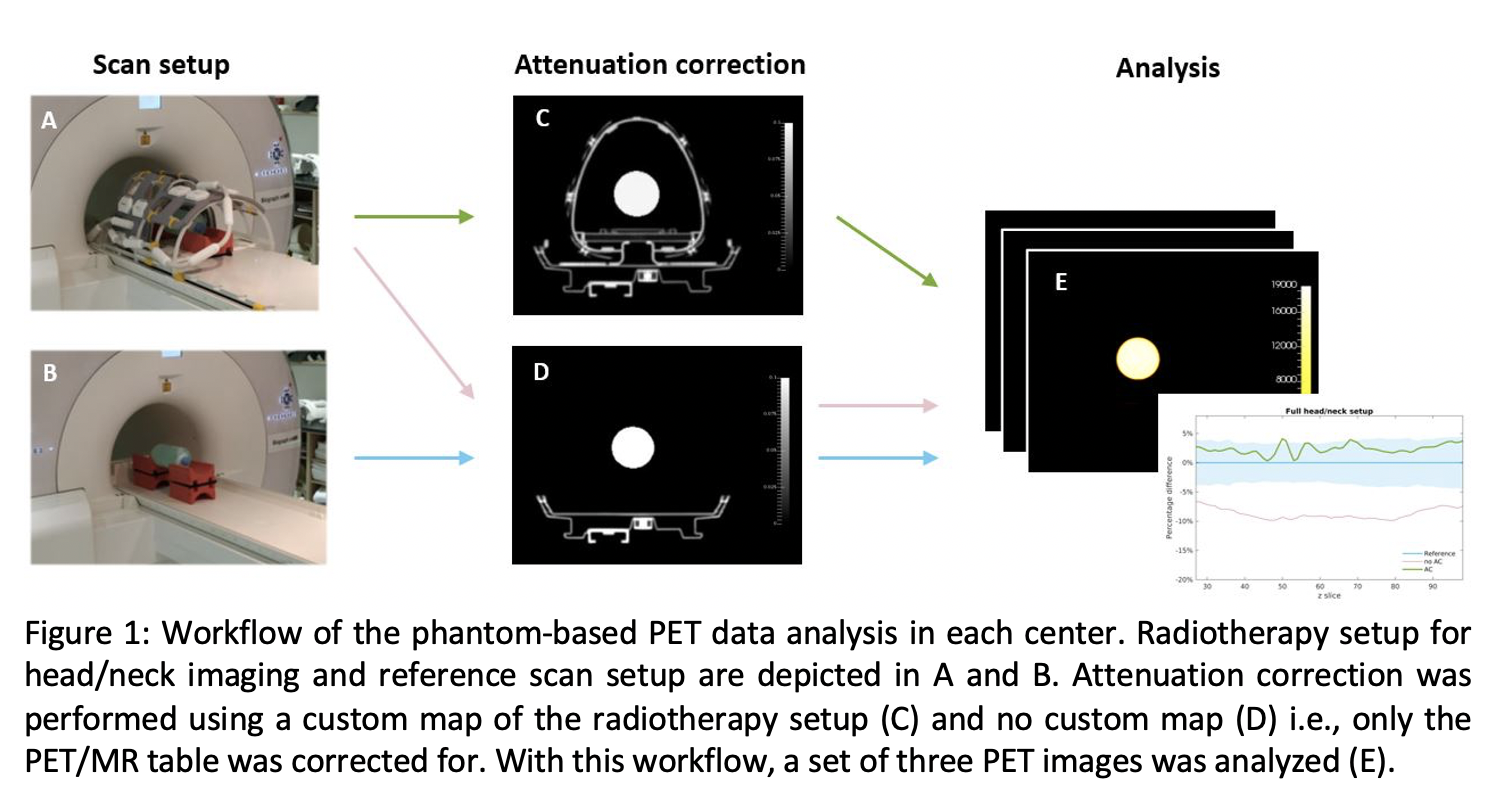
Results
PET data corrected with custom attenuation maps reduced the bias in quantitative image values measured with the radiotherapy setup from -8.7% to 2.4% on average in center 1, and from -9.9% to 1.0% in center 2 (Fig2). In both centers, the final bias was within the error of the reference measurement (< 1 SD). It was similar to the repeatability error determined in the repeated reference scans which was 1.1% and 2.0% in the two centers. Consistent with the phantom results, activation of the custom attenuation maps in PET reconstruction of the patient data up-corrected the local PET SUV values in the primary tumor and PET positive lymph nodes by 11% on average.
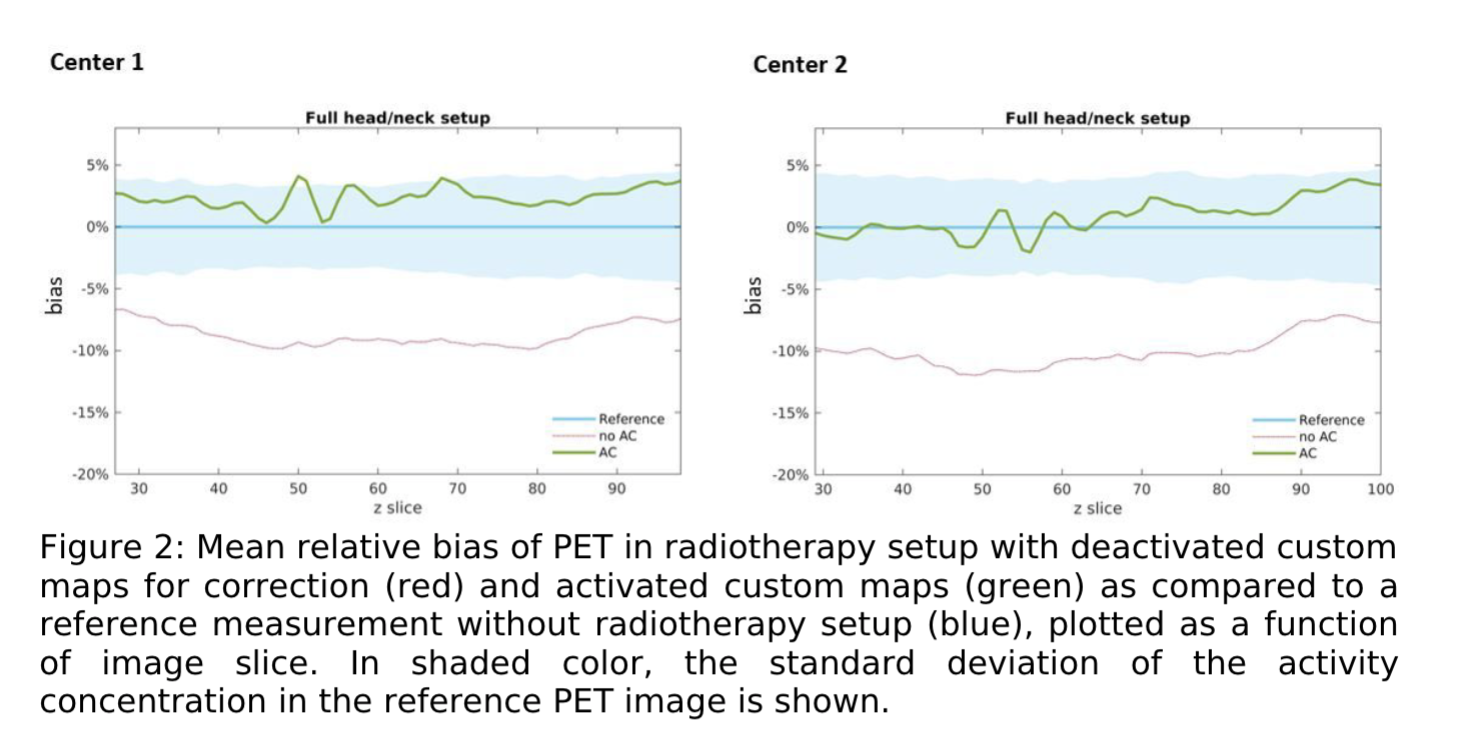
Conclusion
Custom attenuation correction of radiotherapy hardware setups for combined PET/MRI is necessary for quantitative PET. Good reproducibility of custom corrected PET data has been demonstrated and the presented workflow may serve as a quality assurance (QA) program for multicenter imaging trials.