Application of BioXmark® liquid fiducial markers for CyberKnife® stereotactic body radiotherapy
PO-1608
Abstract
Application of BioXmark® liquid fiducial markers for CyberKnife® stereotactic body radiotherapy
Authors: Indra Surkova1, Gaļina Boka1, Māris Mežeckis1, Sandra Lediņa1, Vladyslav Buryk1
1Radiosurgery Centre Sigulda, Radiation Oncology, Sigulda, Latvia
Show Affiliations
Hide Affiliations
Purpose or Objective
Robotic stereotactic body radiotherapy (SBRT) has the benefit of automatic tracking of moving targets. Fiducial markers are essential for multiple target locations, but implantation of fiducials are linked with risk of complications. The BioXmark® is a novel liquid based fiducial marker which might be implanted with a very thin needle reducing possible risk of complications. Earlier BioXmark has been tested for image guided radiotherapy with conventional linear accelerators, but has not yet been tested for automatic tracking with CyberKnife® robotic SBRT systems.
The purpose of this study is to compare BioXmark® and current standard solid gold fiducial markers in CyberKnife® tracking system, in order to evaluate correction precision during the CyberKnife® stereotactic body radiotherapy (SBRT).
Material and Methods
BioXmark® fiducials (Nanovi A/S, Lyngby, Denmark) were prepared in vitro with following volumes 50 μL, 100μL, and 200 μL and cooled to acquire stability of the markers. 4 fiducials of each size were placed and sealed in custom-made inserts according to Accuray recommendations (≥20mm from each other). Following inserts were placed in the anthropomorphic E2E head and neck phantom (Cetacea®, California, USA) with built-in standard gold markers. Three experimental treatment plans with every fiducial size were made with 3 image centers and 8 fiducials in each (4 built in standard gold fiducials and 4 test fiducials). Measures were made in 3 different phantom positions and systems proposed corrections were recorded. The average value of linear, rotational offsets and “uncertainty” parameters were assessed to characterize accuracy. Significant difference between BioXmark® and reference gold fiducials was considered as ≥0.5mm in translations and ≥0.5 deg in rotations (W. Kilby et al. 2010).
Results
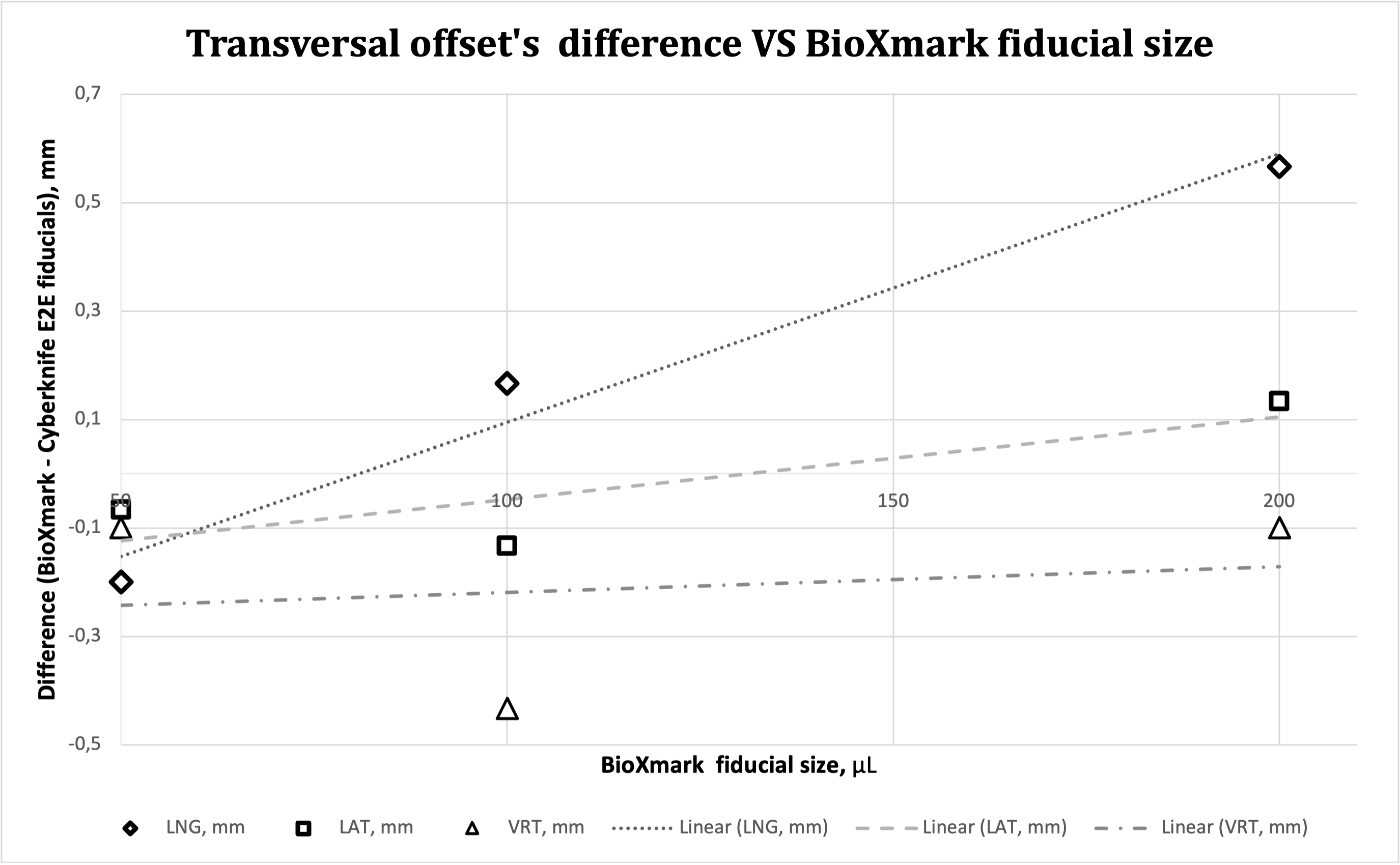
All BioXmark® fiducials were recognized by Cyberknife® tracking system. Longitudinal, vertical and lateral offsets presented by 50μL, 100μL BioXmark® fiducials compared to standard metal markers showed minor, insignificant differences. However 200 μL BioXmark® fiducials longitudinal offset was 0.57 mm which is considered as significant (Table 1, Graph 1). Differences in rotational offsets showed uncertain results, that might be affected by uncertainty error which was higher for BioXmark® compared to reference fiducials.
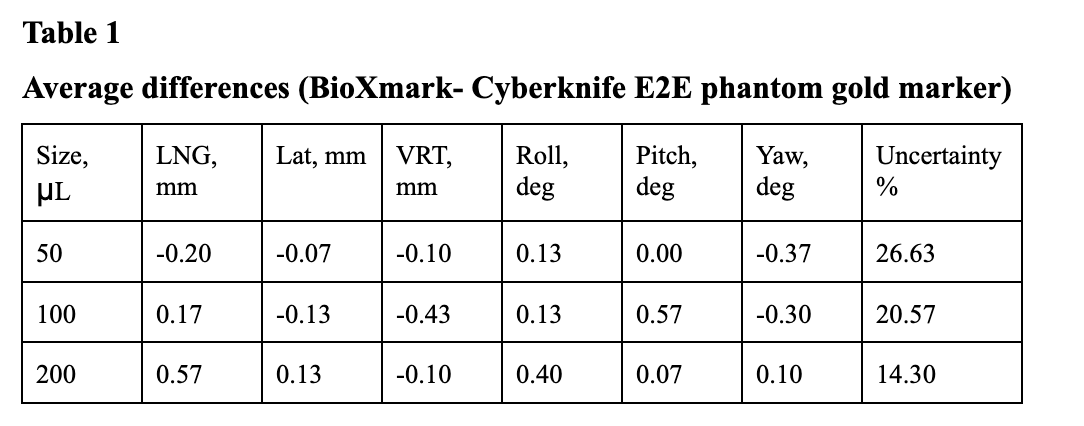
Graph 1
Conclusion
The BioXmark® can be recognized and followed by CyberKnife® tracking system, however significant differences were observed compared to standard fiducials. Smaller volume of the fiducial tend to show smaller errors compared to reference fiducials, however more measurements are needed to show statistically significant results. Additional research with smaller marker volumes and dosimetric films is required for more precise evaluation of tracking abilities of BioXmark® fiducials prior to clinical application.