Alanine dosimetry: an easy in vivo validation of irradiation of subcutaneous murine tumors
Christina Ankjærgaard,
Denmark
PO-1527
Abstract
Alanine dosimetry: an easy in vivo validation of irradiation of subcutaneous murine tumors
Authors: Christina Ankjærgaard1, Astrid Z. Johansen2, Maria Perez-Penco2, Claus E. Andersen1, Daniel H. Madsen2, Claus P. Behrens3
1Technical University of Denmark, Health Technology, Roskilde, Denmark; 2Copenhagen University Hospital - Herlev and Gentofte, National Center for Cancer Immune Therapy, Copenhagen, Denmark; 3Copenhagen University Hospital - Herlev and Gentofte, Dept. of Oncology, Copenhagen, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
Studies using mouse cancer models are important to aid the translation of preclinical radiotherapy research to clinical implementation. Ankjærgaard et al., (2021 in Radiat. Meas. 147, p. 106636) demonstrated alanine to be an excellent in vivo dosimeter for murine subcutaneous flank tumors. Mice were positioned on their side, resulting in the contralateral side receiving approximately 3.5% (0.5 Gy) of the prescribed dose, which could be problematic for investigation of abscopal effects. Here we investigate a different setup, where mice are positioned on the stomach during irradiation. This position allows for irradiation of the hind leg and a larger distance between subcutaneous tumors in both flanks. The aim is to test whether the irradiation setup 1) can be used to irradiate subcutaneous hind leg tumors and 2) provides enhanced tissue sparing to the contralateral flank.
Material and Methods
Seven C57BL/6 female mice (weights: 21.6 - 24.4
g) were anaesthetized using air with 2.8-4% isoflurane, positioned on their stomach and irradiated in
two separate sessions with 1) 15 Gy to the right hind leg with an alanine
pellet placed directly on the skin by the ankle, and 2) 11 x 15 Gy (=165 Gy) to
the right flank with alanine pellets placed on both flanks. The large dose
ensures measurable signal in the contralateral alanine pellet. Pellets were placed
at the isocenter of a 3x3 cm2 10 MV FFF asymmetrical field delivered
by a Varian TrueBeam LINAC, with the main part of the field outside the mouse
to spare normal tissue at the contralateral side. Mice were covered in bolus on
all sides, leaving minimal air between mice, alanine and bolus.
Results
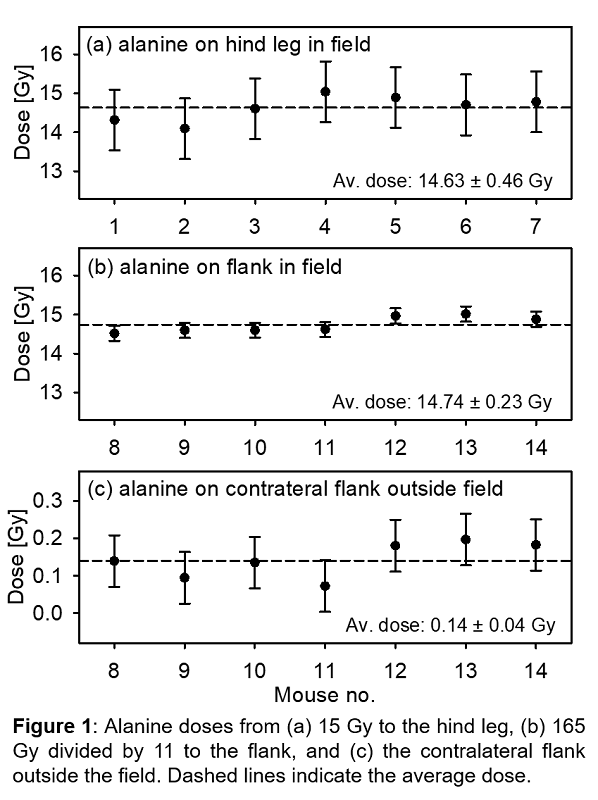
The measured average dose to
the hind legs was (14.63 ± 0.43) Gy, and to the right flank (14.74 ± 0.23) Gy
(Fig. 1), both at k=2, and in
agreement with previously published data. The dose is about 2% lower than the
planned 15 Gy, likely caused by the TPS dose was calculated from an assumption
of dose deposited in water, not taking a possible small air gap between mouse
and bolus into account, a possibly lower output of the LINAC on the day (it can
vary ±2%), and setup uncertainties. The average dose from the pellets placed on
the contralateral flank (Fig. 1c) gave (0.14 ± 0.04) Gy at k=2, which is approximately 1% of the prescribed dose. This is an
improvement over the previous setup, giving a dose reduction of about 70%. This
approach of using alanine as an in
vivo
dosimeter on live mice will be extended to proton-, electron- and l energy
x-ray beams.
Conclusion
This work presents alanine
dosimetry for validating a simple setup for small-field localized irradiations
of subcutaneous tumors implanted in the flank or in the hind limbs in mice. The
setup has a large degree of reproducibility between irradiations, and spares
the contralateral side of the mouse, allowing investigation of abscopal effects.
Alanine is easy and flexible to use, its size comparable to subcutaneous mouse tumors,
which makes it ideal for dosimetric validation in vivo studies.