Low-dose RT for benign musculoeskeletal disorders:clap your hands, stomp your feet,return to be fit!
PO-1442
Abstract
Low-dose RT for benign musculoeskeletal disorders:clap your hands, stomp your feet,return to be fit!
Authors: Beatriz Álvarez1, Ángel Montero2, Rosa María Alonso3, Jeannette Josefina Valero1, Raquel Ciérvide1, Mercedes López1, Leyre Alonso4, Emilio Sánchez1, Mariola García-Aranda1, Xin Chen3, Ovidio Hernando3, Carmen Rubio1
1Hospital Universitario HM Sanchinarro. HM Hospitales, Radiation Oncology, Madrid, Spain; 2Hospital Universitario HM Sanchinarro. HM Hopsitales, Radiation Oncology, Madrid, Spain; 3Hospital Universitario HM Puerta del Sur. HM Hospitales, Radiation Oncology, Madrid, Spain; 4Hospital Universitario HM Sanchinarro. HM Hospitales, Medical Physics, Madrid, Spain
Show Affiliations
Hide Affiliations
Purpose or Objective
Do you imagine having
hands or feet pain every day? Inflammatory and degenerative musculoeskeletal
disorders (MSD) of hand and feet are
common causes of pain and functional disability in western countries and there
is still no definitive cure. Herein, we present clinical outcomes of 127 with
hand or feet degenerative/inflammatory disorders undergoing LDRT for symptomatic
pain and functional relief.
Material and Methods
Between
April 2015 and August 2021, 127 patients (25 men
and 102 women) with a median age 51 years-old were prospectively enrolled.
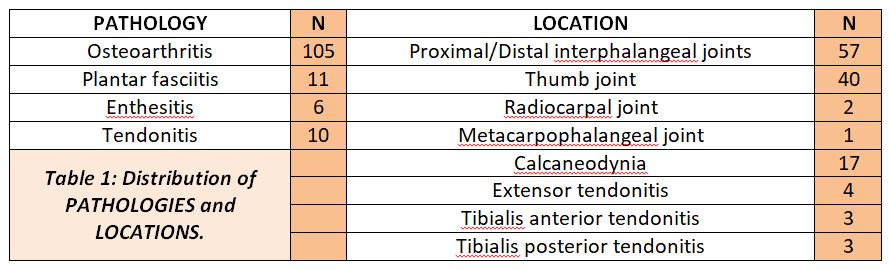
(Table 1)
LDRT comprised of 6 fractions of 0.5-1 Gy
on every-other day up to a total dose of 3-6 Gy. Clinical response was
evaluated according to the visual analogic score (VAS) for pain level and to
the von Pannewitz score (VPS) for joint functionality. Those patients not
reaching subjective adequate pain relief after 12 weeks from treatment were
offered a second identical LDRT course.
Results
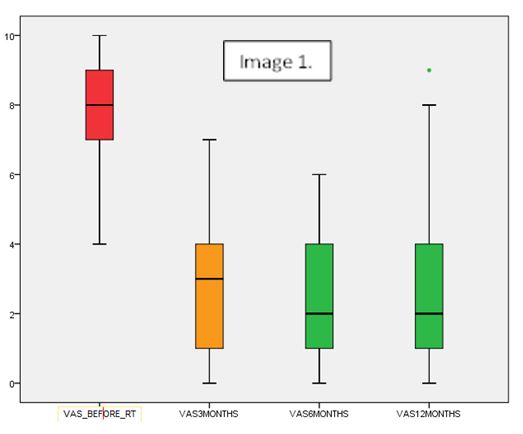
With a median follow-up of 10 months
(range 1-43) and starting with a median VAS before treatment of 8 (range 3-10),
80% of the patients referred improvement of the pain describing median VAS at
3, 6 and 12 months of 4 (range 0-9), 2 (range 0-9) and 2 (range 0-9),
respectively, being this reduction statistically significant (p<0.001)
(Image 1). Eighty-five patients (67%) needed a second course of treatment at a
median time interval of 13.6 weeks (range 7.4-21).
70% of patients reported functionality
improvement after LDRT according to von Pannewitz score.
In the univariant analysis we did not find
any differences between variables.
No acute or late complications were
observed.
Conclusion
LDRT appears to be safe
and useful for hand and feet degenerative/inflammatory
MSD associating good rates of pain relief and functionality improvement
without treatment related toxicities. However, further studies are necessary to
confirm these promising results.