SBRT Boost for Intermediate and High-Risk Prostate Cancer. Results of a single Chilean institution
María Fernanda Sánchez,
Chile
PO-1387
Abstract
SBRT Boost for Intermediate and High-Risk Prostate Cancer. Results of a single Chilean institution
Authors: María Fernanda Sánchez1, Federico Bakal1, Alexis Troncoso2, Piero Bettoli1
1Fundación Arturo López Pérez, Department of Radiation Oncology, Santiago, Chile; 2Universidad de Chile, Departamento de Tenología Médica, Santiago, Chile
Show Affiliations
Hide Affiliations
Purpose or Objective
Treatment of intermediate and high-risk prostate cancer with a low dose rate (LDR) or high dose rate (HDR) brachytherapy given as a boost has been shown to increase biochemical progression free survival (bPFS). Despite this, many patients are not candidates for the procedure, either because the patient prefers to avoid an invasive procedure or they have no access to this technique (a major problem in Latin America). The purpose of the study is to evaluate the use of stereotactic body radiotherapy (SBRT) as a boost in prostate cancer.
Material and Methods
Intermediate and high-risk prostate cancer patients within our SBRT boost protocol were prospectively assessed. For intermediate risk patients a ≥ 15% lymph node risk (according to MSKCC nodal risk nomogram) was mandatory for inclusion. All patients were treated with SBRT boost to the prostate (21 Gy given in 3 fractions) followed by IMRT directed to prostate, seminal vesicles and pelvic lymph nodes (46 Gy in 23 fractions). Androgen deprivation hormonal therapy (ADT) was administered as per physician’s discretion. Biochemical disease-free survival (bDFS) was calculated using the Phoenix definition. Acute and late genitourinary (GU) and gastrointestinal (GI) toxicities were graded using CTCAE version 5.0.
Results
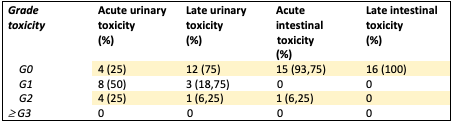
After a median follow-up of 31 months (IQR 8.2-39) sixteen patients were eligible for analysis. Median age of the cohort was 60 years (IQR 60-72). Median prostate volume was 46 cc (IQR 30-70) and median baseline IPSS score 10.5 (IQR 6-15). Two (12.5%) and 14 patients (87.5%) were classified as intermediate and high-risk group according to the NCCN risk classification respectively. 43.8% (n=7) and 25% (n=4) of the patients received 24 and 12 months of ADT respectively; 25% (n=4) received 6 months and only one patient did not receive ADT. No relapses were seen during follow-up. Actuarial 3-year bDFS was 100% for the entire cohort. Treatment was well tolerated with no acute or late Grade ≥ 3 GI or GU toxicities. Few patients experienced acute GI or GU Grade 2 toxicities (4 and 1 patients respectively). Late GU Grade 2 complications were observed only in 1 patient. No late Grade 2 GI complications were observed.
Table 1 Baseline Characteristics
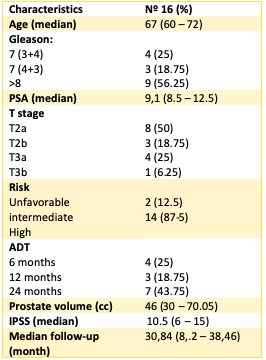
Table 2 Acute and Late CTCAE toxicity for genitourinary and gastrointestinal systems
Conclusion
SBRT boost for intermediate and high risk prostate cancer patients is feasible, well-tolerated and reveals excellent early bDFS. This promising treatment approach could be an excellent alternative for patients who are not candidate, decline or have no access to a brachytherapy boost, nevertheless further follow-up is needed.