Accelerated hypofractionation with SIB-IMRT in Anal Cancer : assessment of efficacy and toxicity
PO-1324
Abstract
Accelerated hypofractionation with SIB-IMRT in Anal Cancer : assessment of efficacy and toxicity
Authors: Sara Lucidi1, Niccolò Bertini1, Mauro Loi2, Pierluigi Bonomo2, Giulio Francolini2, Daniela Greto2, Gabriele Simontacchi2, Alessandra Galardi2, Livia Marrazzo3, Andrea Allegra4, Barbara Guerrieri4, Erika Scoccimarro4, Matteo Mariotti4, Maria Grazia Carnevale4, Giulia Stocchi4, Lucia Pia Ciccone4, Anna Peruzzi4, Victoria Lorenzetti4, Cinzia Talamonti3, Stefania Pallotta3, Monica Mangoni4, Lorenzo Livi4
1University of Florence, Department of Biomedical, Experimental, and Clinical Sciences M. Serio, Florence, Italy; 2Azienda Ospedaliero-Universitaria Careggi, Radiation Oncology, Florence, Italy; 3Azienda Ospedaliero-Universitaria Careggi, Medical Physics Unit, Florence, Italy; 4University of Florence, Department of Experimental and Clinical Biomedical Sciences '' M. Serio'', Florence, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
The historical standard of care for the curative treatment of squamous cell carcinoma of the anal canal (SCCAC) is definitive chemoradiation in conventional fractionation. The introduction of modern radiotherapy techniques such as Tomotherapy allowed for the implementation in this setting of Simultaneous Integrated Boost (SIB) using slightly hypofractionated schedules. This may result in more focused irradiation with reduced overall treatment time (OTT), although concerns for increased toxicity and impaired locoregional control (LRC) have been raised. The aim of our work was to retrospectively evaluate LRC, Distant Metastasis Free Survival (DMFS), and toxicity in patients with SCCAC treated with SIB – intensity-modulated radiation therapy (IMRT).
Material and Methods
We retrospectively reviewed patients with locally advanced SCCAC treated with curative intent in our institution with Tomotherapy between May 2014 and August 2018. A total dose of 55 Gy to the primary tumor and macroscopically involved lymph nodes and 45 Gy to elective lymph node areas, were delivered in 25 fractions of 2.2 and 1.8 Gy respectively, in 5 weeks OTT. Concurrent chemotherapy (CT) was administered with Fluoropyrimidin plus Mitomycin (MMC). Acute and late toxicity was scored according to CTCAE v.5. Statistical analysis was performed to identify variables correlated with outcome and toxicity.
Results
Fifty-two patients were included with a median follow-up of 50 months (range: 5–109). Eight patients were current smokers (15%). Tumor stage was T≥3 in 17 (33%) patients. Nodal stage was N2 or N3 in 18 (35%) patients. Volume delineation was simulation PET-assisted in 34 (65%) patients. Concurrent CT was prescribed to 36 patients (69%). The 3-year LRC rate was 88%: both active smoking and omission of concurrent CT were independently associated with impaired LRC (p=0.028 and p=0.034 respectively) at multivariate analysis (Fig.1). The 3-year DMFS rate was 86%: nodal staging ≥N2 and treatment discontinuation >5 days negatively impacted on DMFS at univariate analysis (p=0.0008 and p=0.029, respectively), although only nodal staging ≥N2 was correlated to a poorer outcome at multivariate analysis (p=0.012) (Fig.2). The rate of radiation dermatitis, diarrhea and cystitis were 76%, 31%, and 4% for >G2 toxicity and 42%, 7% and 0% for >G3 toxicity, respectively. Acute >G3 dermatitis was associated with CT (p=0.047) and tumor stage ≥3 (p=0.0025): in all cases, dermatitis regressed after the end of treatment. No G>3 chronic sequelae were observed.
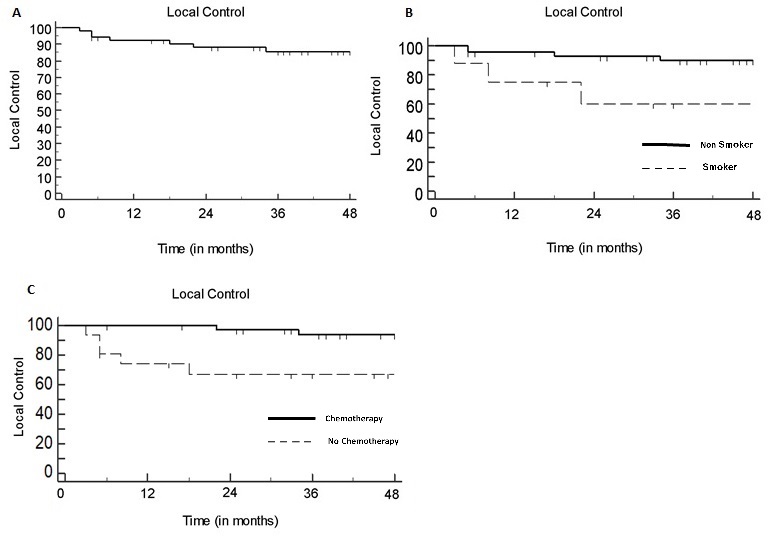
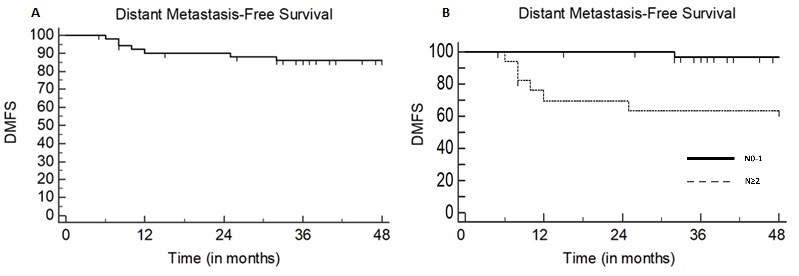
Conclusion
Our results support the feasibility of SIB-IMRT in treatment of SCCAC, with acceptable toxicity incidence and favorable outcome results. Active smoking and omission of chemotherapy negatively impacted LRC, while N staging correlated with metastatic relapse risk. Increased severe skin toxicity was associated with the primary tumor stage, requiring careful treatment surveillance in patients with T3-4 disease.