Charlson Comorbidity Index for patients selection in definitive chemoradiation for anal carcinoma
PO-1299
Abstract
Charlson Comorbidity Index for patients selection in definitive chemoradiation for anal carcinoma
Authors: Sara Lucidi1, Andrea Romei1, Niccolò Bertini1, Mauro Loi2, Pierluigi Bonomo2, Isacco Desideri3, Vanessa Di Cataldo4, Beatrice Detti3, Marta Casati5, Margherita Zani5, Chiara Arilli5, Viola Salvestrini1, Manuele Roghi1, Michele Aquilano1, Michele Ganovelli1, Marco Banini1, Marianna Valzano1, Chiara Bellini1, Icro Meattini1, Lorenzo Livi1
1University of Florence, Departments of Biomedical, Experimental, and Clinical Sciences ''M. Serio'', Florence, Italy; 2Careggi University Hospital, Radiation Oncology , Florence, Italy; 3Careggi University Hospital, Radiation Oncology, Florence, Italy; 4Florentine Institute of Care and Assistance, IFCA Radiotherapy and Medical Physics Unit, Florence, Italy; 5Careggi University Hospital, Department of Medical Physics, Florence, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
The standard of care for the curative treatment of squamous cell carcinoma of the anal canal (SCCAC), is definitive chemoradiation (CRT): however, this treatment may be burdened with severe side effects, and some patients may not be able to complete the planned treatment. In order to tailor the optimal treatment intensity, criteria for patients selection are needed to identify those who may achieve the largest benefit from CRT, whereas avoiding undue toxicity in frail subjects. The aim of our study is to evaluate prognostic variables correlated with clinical outcomes in patients treated with CRT.
Material and Methods
A consecutive cohort of patients with SCCAC treated with Simultaneous Integrated Boost – Intensity Modulated Radiation Therapy (SIB-IMRT) in our institution between 2014 and 2018 was analyzed. Clinical information including age-adjusted Charlson Comorbidity Index (CCI), treatment schedule and dosimetric data were collected. Endpoints were Progression-Free Survival (PFS), Cancer-Specific Survival (CSS) and Overall Survival (OS). Statistical analysis was performed to correlate clinical and treatment-related variables with outcomes.
Results
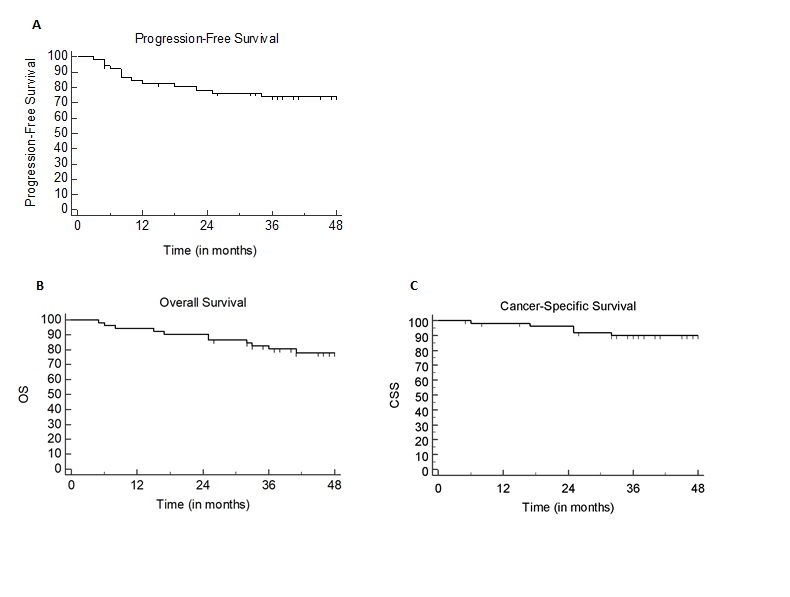
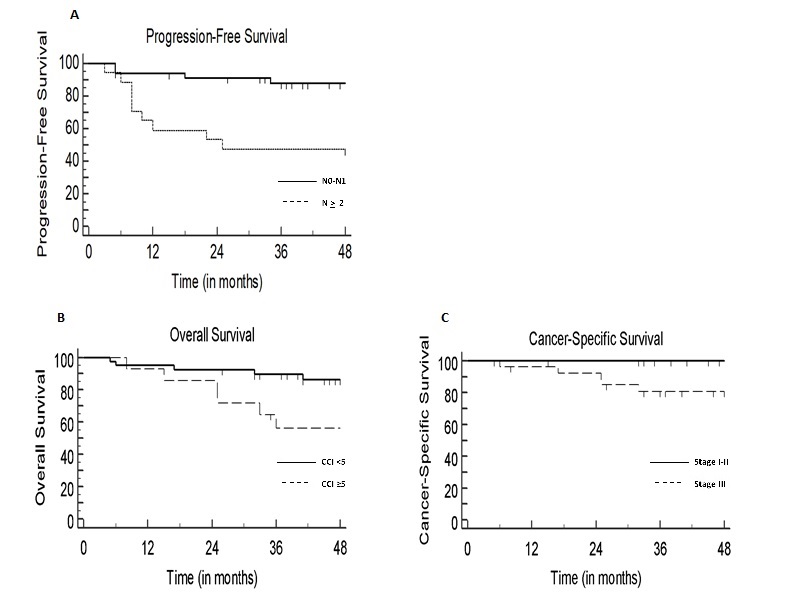
Fifty-two patients were included in our analysis: 15 (29%) patients were men and 36 (71%) were women. Median age was 66 years (range 39-86 years). Fourteen patients (27%) had a CCI > 5. Disease stage was > 3 in 17 (33%) patients. Median size of PTV receiving 55 Gy (PTV 1) was 250cc. All patients achieved treatment completion to a final dose of 55 Gy in 2.2 Gy/fraction to the tumor and involved lymph nodes and 45 Gy in 1.8Gy/fraction to elective drainages in 25 fractions. Thirty-six patients (70%) received concurrent chemotherapy. At a median follow-up of 50 months (range 5-109 months), 6 (12%) and 14 (27%) patients died of disease progression and non-cancer-related events, respectively. No treatment-related deaths were observed. The 3-year PFS, CSS and OS rates were 74%, 89% and 82%, respectively (Fig.1). Nodal staging > 2 and PTV 1 size >250cc were correlated with worse PFS at univariate analysis (p=0.0013 and p= 0.0194, respectively), although only nodal staging > 2 at the multivariate analysis was confirmed as statistically significant (p= 0.023). Stage III disease negatively impacted CSS (p=0.02). Overall, only a CCI > 5 had an impact on OS (p= 0.023) (Fig.2).
Conclusion
Patients who are candidates to definitive CRT for locally advanced SCCAC should be carefully selected based on their age and clinical risk profile, due to the non-negligible occurrences of non-cancer-related deaths. CCI is a simple tool that could be implemented for patient stratification in order to identify patients who are fit for aggressive management.