Stereotactic Body Radiotherapy (SBRT) for liver tumors – An analysis of Dose volume effects
PO-1283
Abstract
Stereotactic Body Radiotherapy (SBRT) for liver tumors – An analysis of Dose volume effects
Authors: Jeevi Selvarajan1, Anil kumar Anand2, Deepak Mittal3, Anil Kumar Bansal2, Naveen Kumawat2
1Max Super Speciality hospital, Radiation Oncology, Delhi, India; 2Max Super Specialty Hospital, Radiation oncology, Delhi, India; 3Max Super Specialty Hospital, radiation oncology, delhi, India
Show Affiliations
Hide Affiliations
Purpose or Objective
Surgical resection is the standard of care for Hepatocellular carcinoma (HCC)and liver metastases, however most patients are unable to undergo surgery due to clinical condition or major cost involved. Stereotactic Body Radiotherapy (SBRT) is a non-invasive technique that enables delivery of ablative high doses of radiation with hypo-fractionation. The advantage over conventional fractionation is that high dose delivered per fraction with reduction of dose to normal tissues, due to rapid dose fall off at the periphery of the target. SBRT can achieve local control in accordance with surgery or other local invasive modalities; a trade off exists between dose delivered, local control, gastrointestinal (GI) complications, hematological toxicities and RILD. Our aim is to analyse the dose volume effects to the hematological and GI toxicity, progression of Child Pugh (CP) score and Radiation Induced Liver Disease (RILD).
Material and Methods
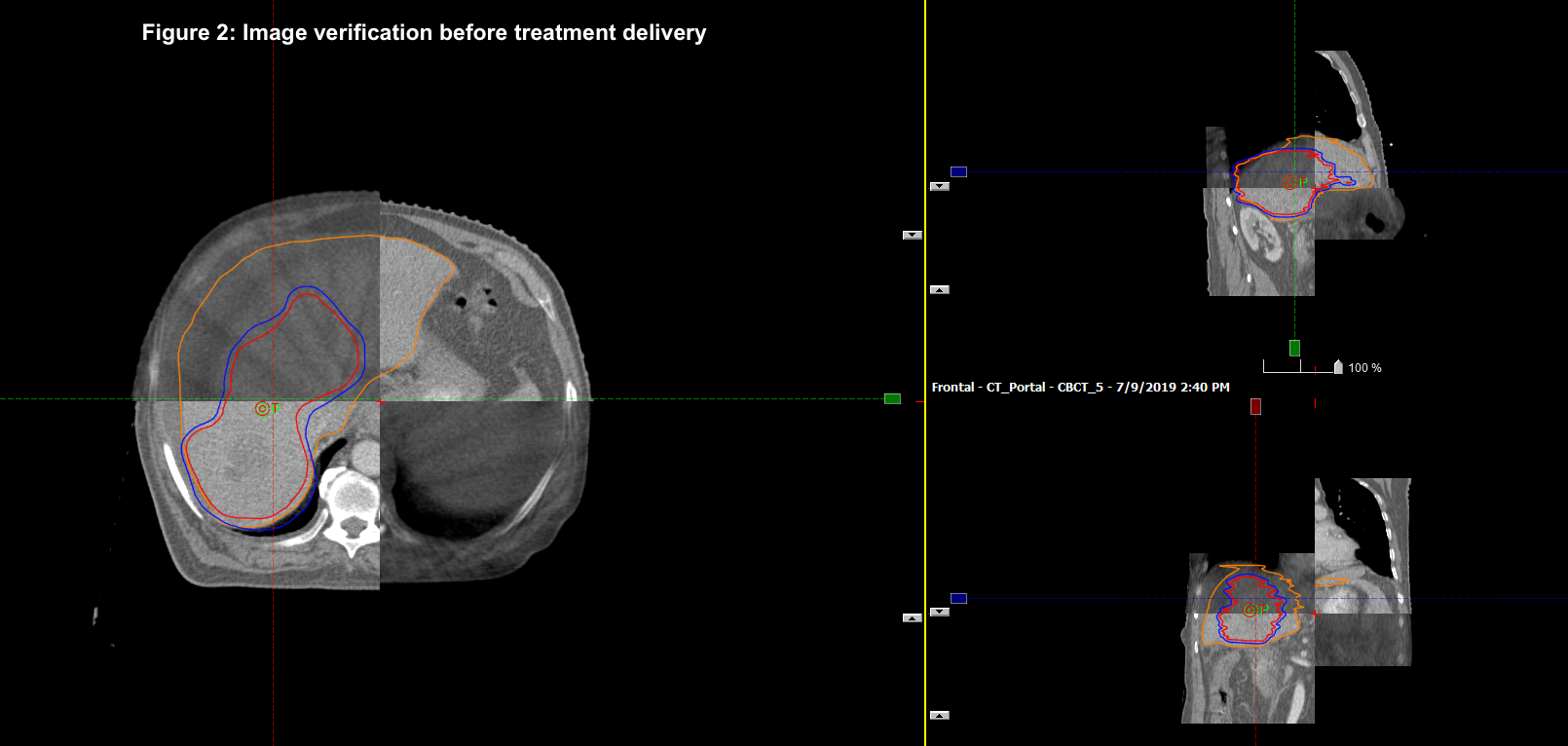
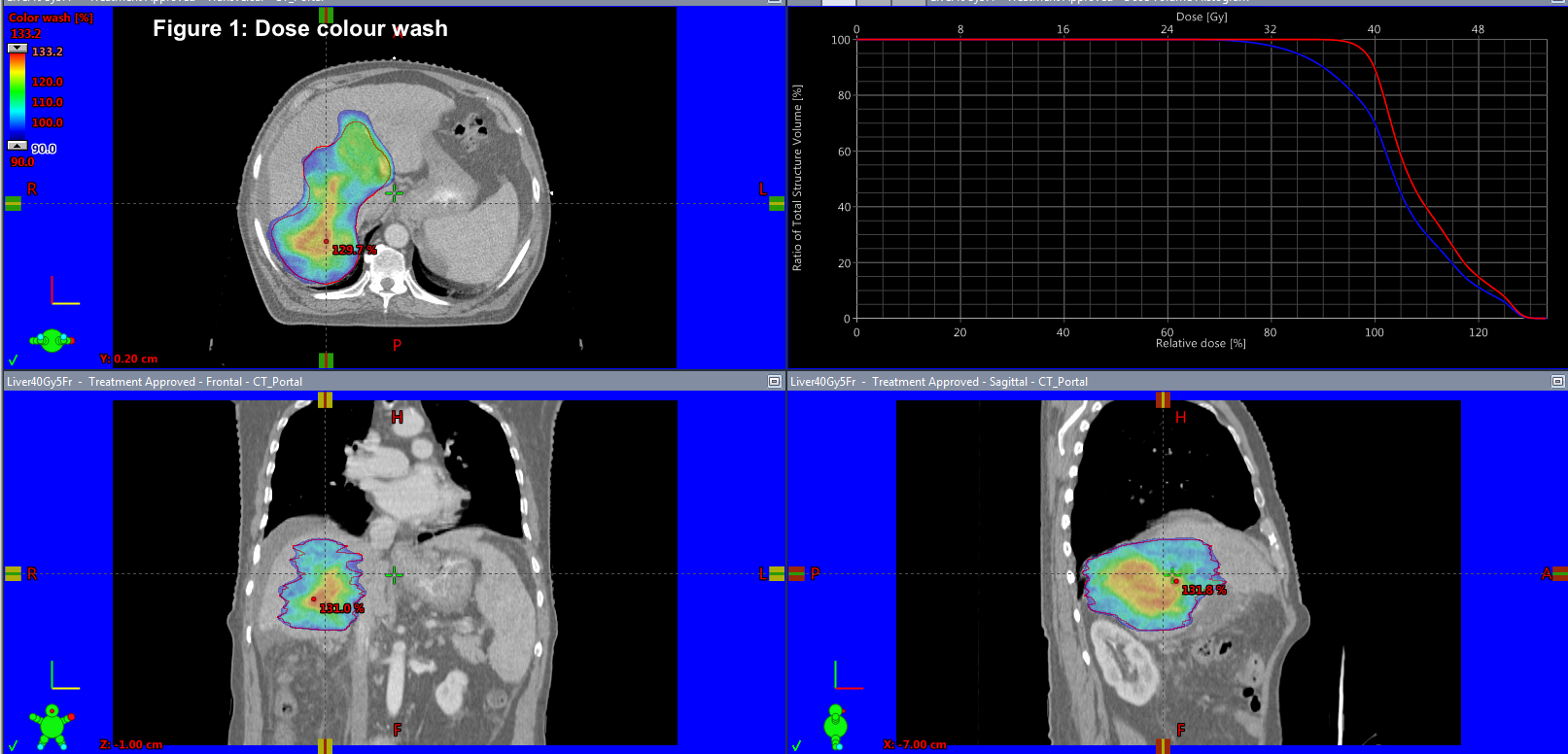
We retrospectively analysed 50 patients treated from 2011 to 2018. HCC was seen in 46 % and metastatic liver tumors 54 %. CP score and LFT were documented. Motion management techniques used were tumor tracking, abdominal compression, gating and 4DCT to account for the movement of the liver and to create differential planning target volume (PTV) margins as per the technique used. Gross tumor volume (GTV) was contoured after discussing with the liver radiologist. Median GTV Volume was 86 cc (6.8 – 867 cc). Median Normal liver volume was 1251 cc (715 – 2700 cc). Median dose delivered was 48 Gy (35- 54 Gy) and median BED10Gy was 85.5 Gy10 (52.2 Gy10to 151.2 Gy10). Median D700 ccof normal liver was 9.4 Gy. The dose was reduced if the tumor was in proximity to the organs at risk or either the D700 cc was exceeding the normal limit.TG 101 was followed for normal tissue constraints.
Results
3 patients lost to follow up. Toxicity analysis and response assessment was by CTCAE V.5 and mRECIST criteria, respectively.
CP Score after SBRT | No.of patients |
A to B | 15 |
A to B progression by ≥ 2 | 5 out of 15 |
B to A | 4 |
No change | 28 |
On analysis, Non classic RILD was observed in 1 patient. No patient had classic RILD in our study. Abdominal pain was seen in 10 %. Nausea/Vomiting was seen in 32% of our patients with significant association with dose per fraction (p=0.009). Grade 2 -3 hematological toxicities were observed in patients more than 50 years of age and higher dose per fraction (> 8Gy/fraction), not statistically significant (p=0.23). Median local control was 8 months with 1-year local control of 42 %. The median overall survival was 12 months and 1-year survival was 72 %.
Conclusion
The uniqueness of delivering SBRT liver is that a fixed dose fractionation regimen is not feasible in all patients due to dose volume effects, D700cc, fear of GI toxicities and RILD. Normal liver volume less than 700 cc remains the strict exclusion criteria. SBRT shall provide better local control rates with BED Gy10more than 70-80 Gy10with acceptable toxicity and no risk of RILD at D700cc < 15-18 Gy.