The base case analysis demonstrated that 2
fractions of SBRT was not cost-effective compared to a strategy of EBRT single
fraction for painful spinal metastases, with an ICER of $194,145/QALY gained. RFA was a dominated treatment strategy (more
costly and less effective) in this model.
In one-way sensitivity analyses, results were most sensitive to
variation of the pain complete response rates after initial treatment with SBRT
and EBRT. Probabilistic sensitivity
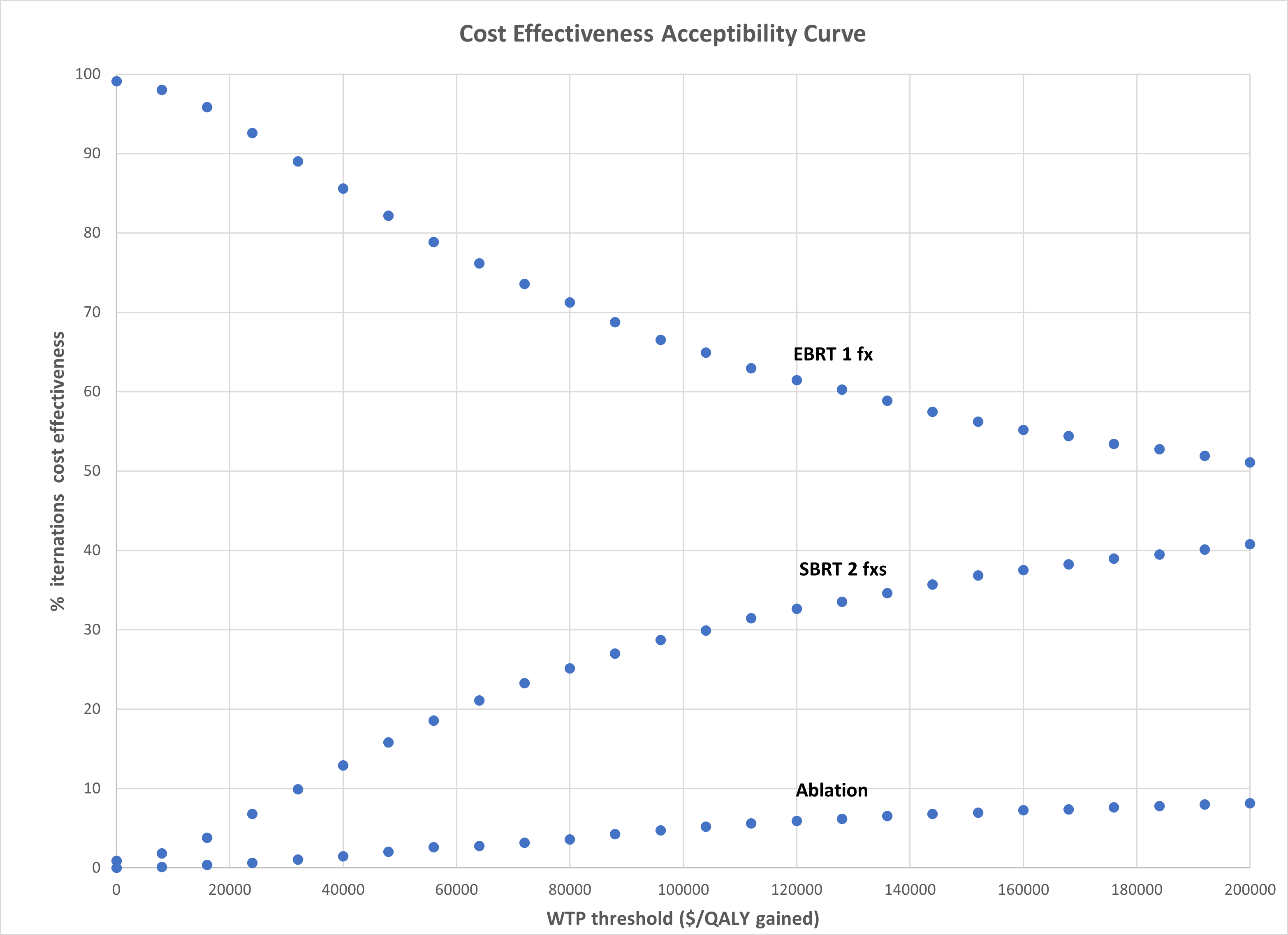
analysis demonstrated that EBRT was favored in 66% of model iterations at a WTP
threshold of $100,000/QALY gained (Figure 1).
In addition, scenario analyses were performed reflecting current
clinical practice. First, EBRT was instead
delivered with 20 Gy in 5 fractions. In
this setting, SBRT approached cost-effectiveness, with an ICER of $139,385/QALY
gained. Next, if median survival were
improved after SBRT, two-fraction SBRT became cost-effective, with ICER of $80,394,
$57,062, and $47,038 for 3, 6, and 9-month improvements in survival. Because two-fraction SBRT data reported 18% of
patients with indeterminant pain response at 3 months, and two-fraction SBRT is
infrequently used in clinical practice, single-fraction SBRT data was also
assessed. Single-fraction
SBRT delivering 24 Gy was cost-effective compared to single-fraction EBRT, with
an ICER of $92,833/QALY gained. Notably, the pain
complete response rate was equivalent in the Sahgal et al. and Sprave et al.
trials when patients with indeterminant pain response were excluded.