Patient-reported acute urinary toxicity after daily adaptive MR-guided SBRT for prostate cancer
Thomas Willigenburg,
The Netherlands
PD-0577
Abstract
Patient-reported acute urinary toxicity after daily adaptive MR-guided SBRT for prostate cancer
Authors: Thomas Willigenburg1, Frederik R. Teunissen1, Joanne van der Velden1, Johannes de Boer1, Jochem van der Voort van Zyp1
1University Medical Center Utrecht, Radiation Oncology, Utrecht, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
While
severe toxicity rates after stereotactic body radiation therapy (SBRT) for the
treatment of prostate cancer (PCa) are low, many patients still experience
moderate toxicity – especially genito-urinary – that can impact quality of life (QoL). The
introduction of magnetic resonance (MR)-guided linear accelerators (MR-Linac)
has enabled daily, high-quality imaging of soft-tissue in addition to daily
re-planning. This potentially increases treatment precision compared to
conventional linacs. However, clinical data on outcomes after 1.5T MR-Linac SBRT
for prostate cancer is still scarce. We present short-term
patient-reported outcomes regarding urinary toxicity in a prospective
cohort of PCa patients treated with MR-guided SBRT.
Material and Methods
PCa
patients treated with 5x7.25 Gy on a 1.5T MR-Linac between March 2020 and May
2021, who participated in the prospective Utrecht Prostate Cohort (UPC) study
(NCT04228211), were included (n=161). Patients with missing International
Prostate Symptom Score (IPSS) data at baseline and/or no follow-up within three
months post-treatment were excluded (n=29). Patients were treated over the
course of 2.5 weeks using an ‘Adapt-to-Shape’ workflow with daily online re-contouring
and re-planning. IPSS questionnaires were filled out at baseline (BL) and 1
month (1M) and 3 months (3M) after treatment. Patients with a clinically
significant increase in IPSS (≥5 points from BL) within three months post-treatment were identified. Descriptive statistics were reported for baseline
characteristics and differences between patients with and without a clinically
significant increase in IPSS were assessed using appropriate statistical tests. IPSS at 1M and 3M was compared to
BL using related-samples Wilcoxon
signed rank test. A
p-value <0.05 was considered statistically significant.
Results
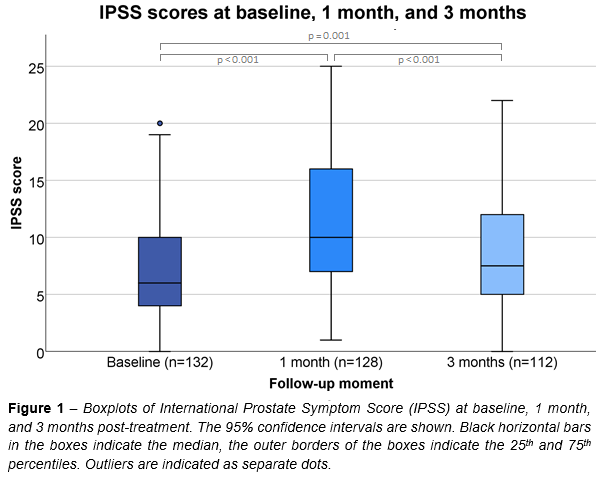
Median
(interquartile range) IPSS was 6 (4-10),
10 (7-16), and 7.5 (5-12) at BL, 1M, and 3M,
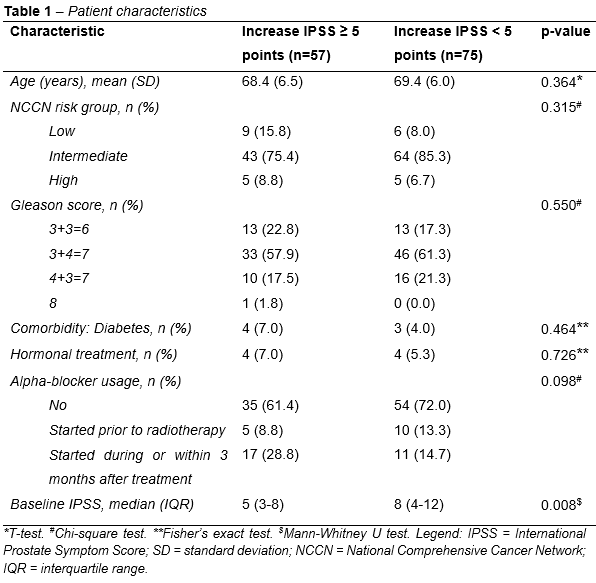
respectively (Figure 1). Median IPSS was significantly higher at 1M and 3M compared to BL, and significantly lower at 3M compared to 1M. Out of 132 patients, 57 (43.2%) reported a clinically relevant increase in IPSS of ≥5
points. Besides a lower median baseline IPSS, no significant differences in
characteristics between those with and without a clinically
relevant increase in IPSS (≥5 points vs <5 points) were observed (Table 1).
Conclusion
The
significant changes in IPSS indicate a notable effect on patient-reported
urinary toxicity in the first three months following MR-guided prostate cancer
SBRT using a 1.5T MR-Linac. A clinically relevant increase in IPSS was observed in a large proportion
of patients, thus leaving room for improvement. Currently, we are investigating
the association between the accumulated dose to the bladder and bladder wall
and patient-reported acute urinary toxicity. This approach is feasible due to the
availability of high-quality 1.5T MR images and treatment plan data for each
fraction.