Feasibility of magnetic resonance-guided stereotactic ablative body radiotherapy of liver cancer
Vikneswary Batumalai,
Australia
PD-0334
Abstract
Feasibility of magnetic resonance-guided stereotactic ablative body radiotherapy of liver cancer
Authors: Maddison Picton1, Vikneswary Batumalai1, David Crawford2, Claire Pagulayan3, Louise Hogan2, Urszula Jelen2, Conrad Loo2, Nicole Dunkerley2, Lori Geddes2, Sandy Sampaio2, Monique Heinke2, Tania Twentyman2, Michael Jameson2, Jeremy de Leon2
1GenesisCare, Radiation Therapy , Sydney, Australia; 2GenesisCare, Radiation Therapy, Sydney, Australia; 3GensisCare, Radiation Therapy, Sydney, Australia
Show Affiliations
Hide Affiliations
Purpose or Objective
Stereotactic ablative
body radiotherapy (SABR) is an effective treatment method for liver cancer. However,
tumour motion and proximity of organs at risk (OAR) can be a limiting factor when
delivering high doses of radiation. Magnetic resonance (MR) guided adaptive
radiotherapy (MRgART) can improve the accuracy and dose coverage of tumour
volumes. This study assessed the feasibility of MRgART for liver cancer.
Material and Methods
Five patients with liver cancer were treated with MRgART. Image sequencing
included a T2 Navigated scan which produced images at expiration. A balance
turbo fast field echo (BTFFE) image was also acquired every fraction to measure
liver motion for the ITV margin. All plans were prescribed to 50Gy in 5
fractions. Plans were adapted in real time for every fraction with treatment
time and dosimetric criteria recorded.
Results
Median patient
age was 61 years (range 54-85 years). Twenty-five
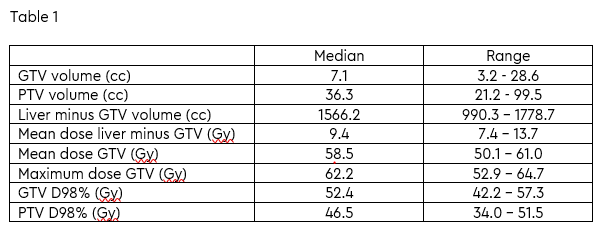
fractions (adapted plans) were delivered to a total of five patients. Dosimetric
parameters are summarised in Table 1. Median dose to 98% of the gross tumour
volume and planning target volume were 52.4 Gy and 46.5 Gy, respectively. OAR
targets were met for all twenty-five fractions. The median time from ‘patient
set-up’ to ‘beam-off’ time was 49.3 minutes (range 39.2-57.0 minutes). All
patients completed treatment with no interruptions.
Conclusion
Our early
experience suggests that MRgART for liver cancer is feasible and safe with
acceptable dosimetric parameters and treatment time. We continue to collect
data and evidence on patient and clinician reported outcome, safety and
tolerability of MRgART.