Predictive factors of late GU toxicity after Cyberknife re-irradiation for locally recurrent PCa
PD-0411
Abstract
Predictive factors of late GU toxicity after Cyberknife re-irradiation for locally recurrent PCa
Authors: Giulio Francolini1, Cecilia Cerbai2, Mauro Loi1, Vanessa Di Cataldo3, Beatrice Detti4, Gabriele Simontacchi1, Lucia Pia Ciccone2, Laura Masi5, Raffaella Doro5, Andrea Allegra2, Viola Salvestrini2, Chiara Mattioli2, Giulio Frosini6, Luca Burchini2, Michele Aquilano2, Giulia Stocchi2, Isacco Desideri2, Lorenzo Livi7
1Azienda Ospedaliero Universitaria Careggi, Radiation Oncology Unit Oncology Department, Florence, Italy; 2University of Florence, Department of Experimental and Clinical Biomedical Sciences "M. Serio", Florence, Italy; 3Istituto Fiorentino di Cura e Assistenza (IFCA), Radiation Oncology CyberKnife Center, Florence, Italy; 4Azienda Ospedaliero Universitaria Careggi, Radiation Oncology Unit, Oncology Department, Florence, Italy; 5Istituto Fiorentino di Cura e Assistenza (IFCA), Department of Medical Physics, Radiation Oncology CyberKnife Center, Florence, Italy; 6 University of Florence, Department of Experimental and Clinical Biomedical Sciences "M. Serio", Florence, Italy; 7University of Florence - Department of Experimental and Clinical Biomedical Sciences "M. Serio", Azienda Ospedaliero Universitaria Careggi - Radiation Oncology Unit Oncology Department, Florence, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
Treatment options for local relapse in prostate cancer after postoperative or definitive radiotherapy (RT) include re-irradiation with stereotactic body RT (re-SBRT). However, dosimetric data allowing to improve late adverse events predictability after this approach are needed. Here we present a retrospective analysis of a cohort of patients treated with re-SBRT through CyberknifeⓇ robotic system, focusing on late genitourinary (GU) toxicity to obtain a model aimed to predict late GU G≥2 adverse events in this population.
Material and Methods
Data about 50 consecutively treated patients from June 2012 to February 2016 were collected. All patients were affected by biochemical relapse defined by European Urology Association Criteria after definitive or postoperative radiotherapy, and macroscopic evidence of intra-prostatic or prostate bed recurrence was detected by 18F-choline PET/CT and MRI. Patients with metastatic or regional nodal disease were excluded. All patients underwent re-SBRT using the CyberKnifeⓇ robotic system, for a total dose of 30 Gy in 5 fractions. Toxicity was assessed by the Common Terminology Criteria for Adverse Events toxicity scale v.4.03. Relationship between late GU G≥2 and Gross Target Volume cubic centimetres (ccGTV), Dose to 50% of urinary bladder volume (DB50), Maximum dose within Planning Target Volume (Dmax), urethra Dmax (UDmax) and Total Equivalent Dose (tEQD2) administered to prostate or prostate bed was explored with logistic regression.
Results
After a median follow up of 48.2 months (6.4-86.3), late G≥2 GU toxicity occurred in 13 (26%) patients. Only one patient experienced grade 3 late GU (hematuria). At univariate analysis, no significant impact of ccGTV (p=0.38), DB50 (p=0.25), Dmax (p=0.88), and tEQD2 total was detected (p=0.76). Only UDmax showed significant association with Late G≥2 GU toxicity (p=0.02). A receive operating characteristic (ROC) curve was used to look for the optimal cut-off point for UDmax that is predictive for late GU adverse events. ROC analysis showed that UDmax≥34.12 Gy best predicted GU toxicity, with a positive and negative likelihood ratio of 3.56 (95% CI 1.1-11.3) and 0.69 (95% CI 0.4-1.1), respectively (AUC 0.66, p=0.06) as reported in figure 1.
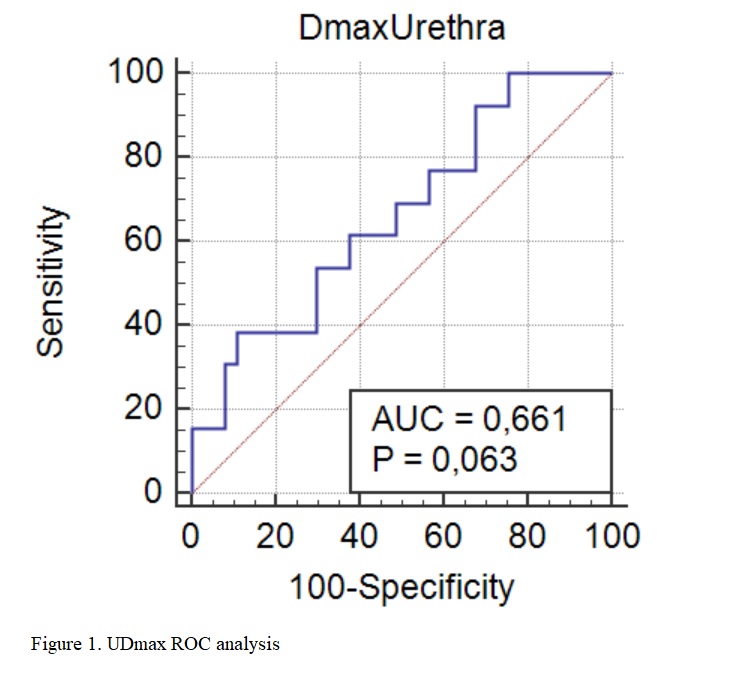
Conclusion
These data suggest that higher urethral dose may be related to higher risk of late G≥2 GU toxicity. Given the low number of events, larger series with long term follow up would be needed to better identify a model useful to predict late GU adverse events after re-SBRT.