Are set-up corrections adequate enough to ensure an optimal dose delivery by IMRT in cancer cervix?
PD-0907
Abstract
Are set-up corrections adequate enough to ensure an optimal dose delivery by IMRT in cancer cervix?
Authors: Pournima Kale1, Pallavi Kalbande1, Nilavarasu Shanmugam1, Bharati Mahindrakar Jain1, Ashok Singh1, Zatin Mathi1, Vinod Hatekar1, Niloy Ranjan Datta1
1Mahatma Gandhi Institute of Medical Sciences, Radiotherapy, Wardha, India
Show Affiliations
Hide Affiliations
Purpose or Objective
IMRT with IGRT followed by brachytherapy and concurrent chemotherapy is usually
the mainstay of treatment in locally advanced cancer cervix (LACC). IMRT plans are
usually generated on snapshot pre-external radiotherapy (ERT) CECT scans taken
1-2 weeks before starting ERT. These IMRT plans are generally considered to be applicable throughout
the entire course of ERT. However, the gross tumor volume (GTV) in LACC is expected
to undergo dynamic regression during the
usual 5 weeks of ERT, representing a sigmoid dose-response curve. The
study aims to analyze the accuracy of pre-ERT IMRT plans on various dose-volume
histogram (DVH) parameters with the transposed GTV on re-CECT taken at 3-4
weeks of ERT.
Material and Methods
15 consecutive patients of LACC were included in this prospective study.
IMRT plans were generated 1-2 weeks before starting ERT to deliver 50Gy in 25 fractions
over 5 weeks to 98% of the planning target volume (PTV). The same IMRT plan was
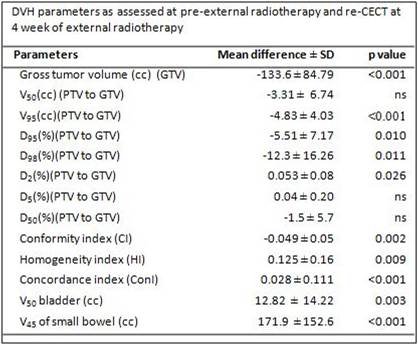
superimposed on a re-CECT taken at 3-4 weeks of ERT. GTV and DVH parameters - V50,
V95, D95, D98, D50, D5,
D2, conformity index (CI), homogeneity index (HI) and concordance
index (ConI) were assessed and compared
between pre-ERT and re-CECT scans. Additionally the V50 of bladder and rectum; V45
and D190cc of small bowel were also evaluated. Before IMRT
delivery, biweekly positional accuracy of the patient setup was monitored using
portal imaging.
Results
A total of 159 shifts in 15 consecutive patients before delivery of
IMRT, each in X, Y and Z axes were recorded. The mean±SD of these shifts in X, Y and Z axes were 0.09±0.5cm, 0.12±0.5cm
and 0.08±0.3cm respectively. Compared to pre-ERT IMRT, significant reduction in GTVs at 3-4 weeks were
observed (p<0.0001). This resulted in significant differences in various DVH
parameters between those of the pre-ERT PTVs
vs. PTVs on re-CECT at 3-4 weeks of ERT V95(p<0.001), D98(p=0.011), CI(p=0.002), HI(p=0.009)
and ConI (p<0.001), V50 bladder
(p= 0.003) and V45 of small
bowel (p<0.001)(Table). The mean differences inV50, D2,
D5 and D50 were insignificant.
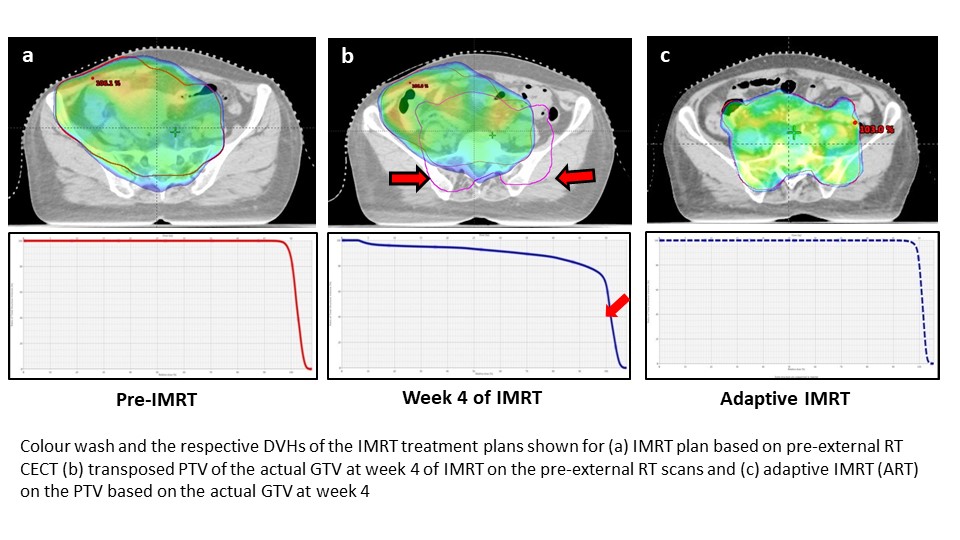
Conclusion
The significant reduction of the GTV in LACC at 4-weeks of ERT results
in a marked variation in the key DVH parameters when compared to the pre-IMRT
plan. Continuing the same pre-ERT IMRT plan for the entire course of 5 weeks of
ERT, would result in inadvertent hot or cold
spots, irradiate higher normal tissue and thus defeat the very purpose of IMRT. Periodic assessment of the dynamic
changes in GTV is thus mandatory by early incorporation of adaptive IMRT plans
to optimize dose to the GTV. This would
ensure adequate intended dose coverage to GTV
that could finally translate
into improved clinical outcomes (Fig).
Thus, IMRT can not only precisely hit the target but could even precisely miss
the target, if tumor regression dynamics are not
timely supplemented by adaptive IMRT plans. Mere setup verifications and their corrections are grossly inadequate
to ensure adequate GTV-PTV coverage as evident on snapshot pre-ERT IMRT plans
in LACC.