Personalized MRI contrast for treatment guidance on an MRI-linac in patients with liver metastases
Robin Navest,
The Netherlands
PD-0896
Abstract
Personalized MRI contrast for treatment guidance on an MRI-linac in patients with liver metastases
Authors: Robin Navest1, Vivian van Pelt1, Tessa van de Lindt1, Tomas Janssen1, Marlies Nowee1, Jan-Jakob Sonke1, Uulke van der Heide1, Petra van Houdt1
1The Netherlands Cancer Institute, Radiation Oncology, Amsterdam, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
MRI-guided stereotactic body radiation therapy (SBRT) is an upcoming local treatment for liver metastases. The visibility of liver metastases during MRI-guided SBRT, however, is unsatisfactory in approximately 30% of patients [1]. Consequently, set-up correction and motion monitoring during treatment are challenging. We therefore propose to personalize the 4D-MRI sequence settings based on patient specific T1 and T2 values of the lesion(s) and surrounding liver tissue.
Material and Methods
23 patients with up to 3 liver metastases received 4D-MRI guided liver SBRT on a 1.5 T Unity MRI-linac (Elekta AB, Stockholm, Sweden). A 4D T2 TSE or 4D balanced GRE was used for set-up correction and motion monitoring. Additionally, before the first treatment fraction a sagittal T1 map (variable flip angle series) and transversal T2 map were acquired for all patients during free breathing.
The mean and standard deviation of T1 and T2 were calculated for ROIs within liver metastases and healthy liver tissue (excluding large vessels). These T1 and T2 values were used to determine the MRI sequence settings that yield optimal contrast between the lesion and surrounding liver tissue, through Bloch simulations. Contrast was defined as the relative difference of the mean MR signal intensity of the lesion ROI with respect to the liver ROI (i.e. C = (SIlesion – SIliver) / SIliver * 100%).
Optimal values of the echo time, refocusing angle and parallel imaging (PI) factor for the 4D T2 TSE and number of startup echoes, flip-angle, PI factor and partial Fourier factor for the 4D balanced GRE were calculated. The optimal 4D-MRI sequences were tested prospectively on a subset of four patients at the end of a treatment fraction. To compare the contrast between the clinically used and optimized 4D-MRI sequences, the absolute ratio between them was calculated (i.e. |Copt / Cclin|).
Results
The measured T1 and T2 values are shown in Fig. 1 for all metastases. The median (range) T1 value of the liver and lesions across all patients were 659 (450-892) and 1105 (578-2466) ms, respectively. T2 values of 58 (40-82) ms for liver and 85 (46-242) ms for metastases were found.
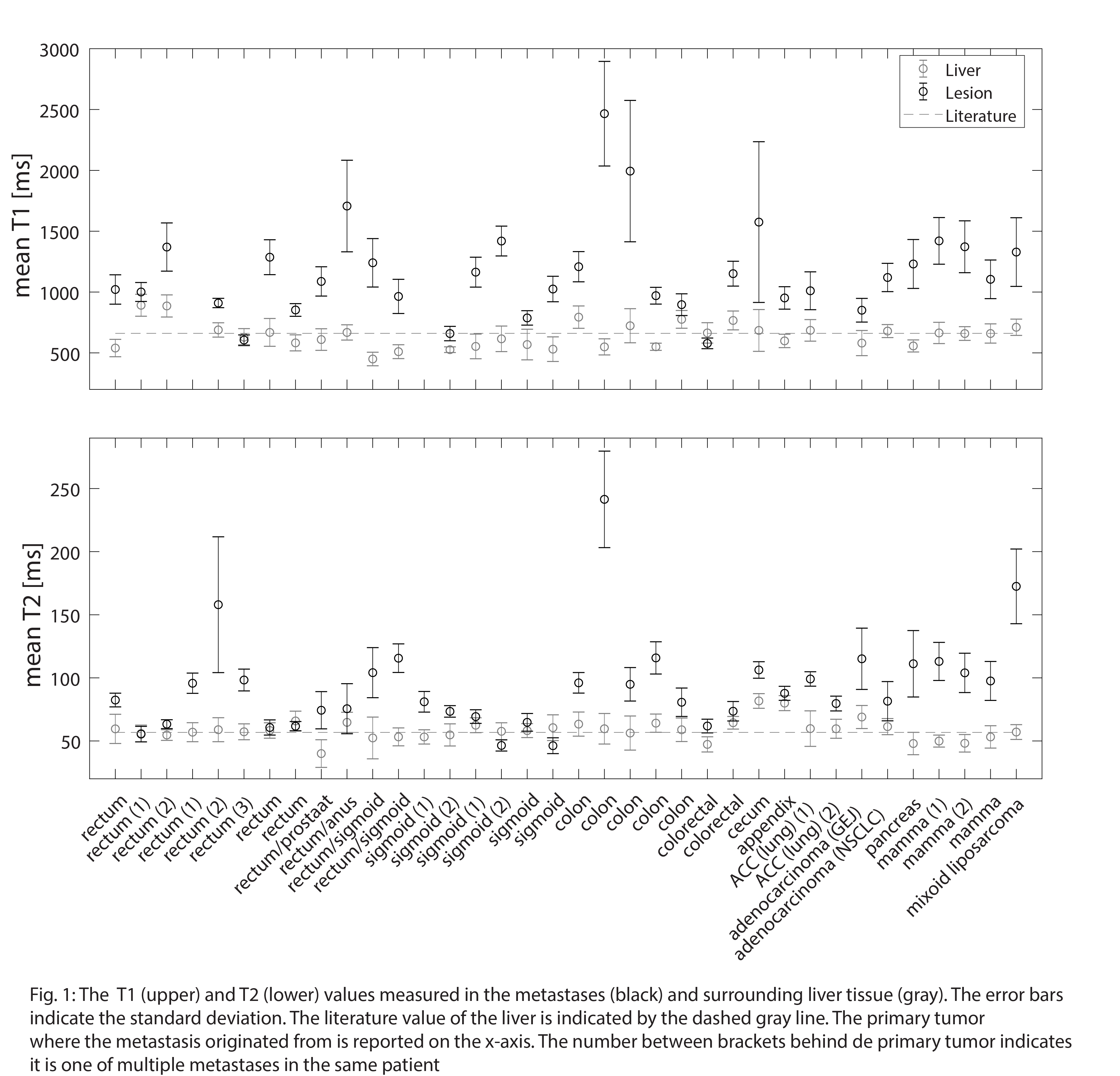
The absolute contrast ratio, and thus lesion visibility, improved for all patients. A minimum and maximum absolute contrast ratio of 1.1 and 4.4 for T2 TSE and 1.02 and 5.9 balanced GRE were found. Fig. 2 shows the lesion visibility improvement of the optimized compared to the clinically used 4D-MR images for the prospective test. Note brighter rim around the lesion in patient 2 and heterogeneous MR signal intensity within the lesion in patient 3 and 4. This makes delineation and contrast estimation more difficult.
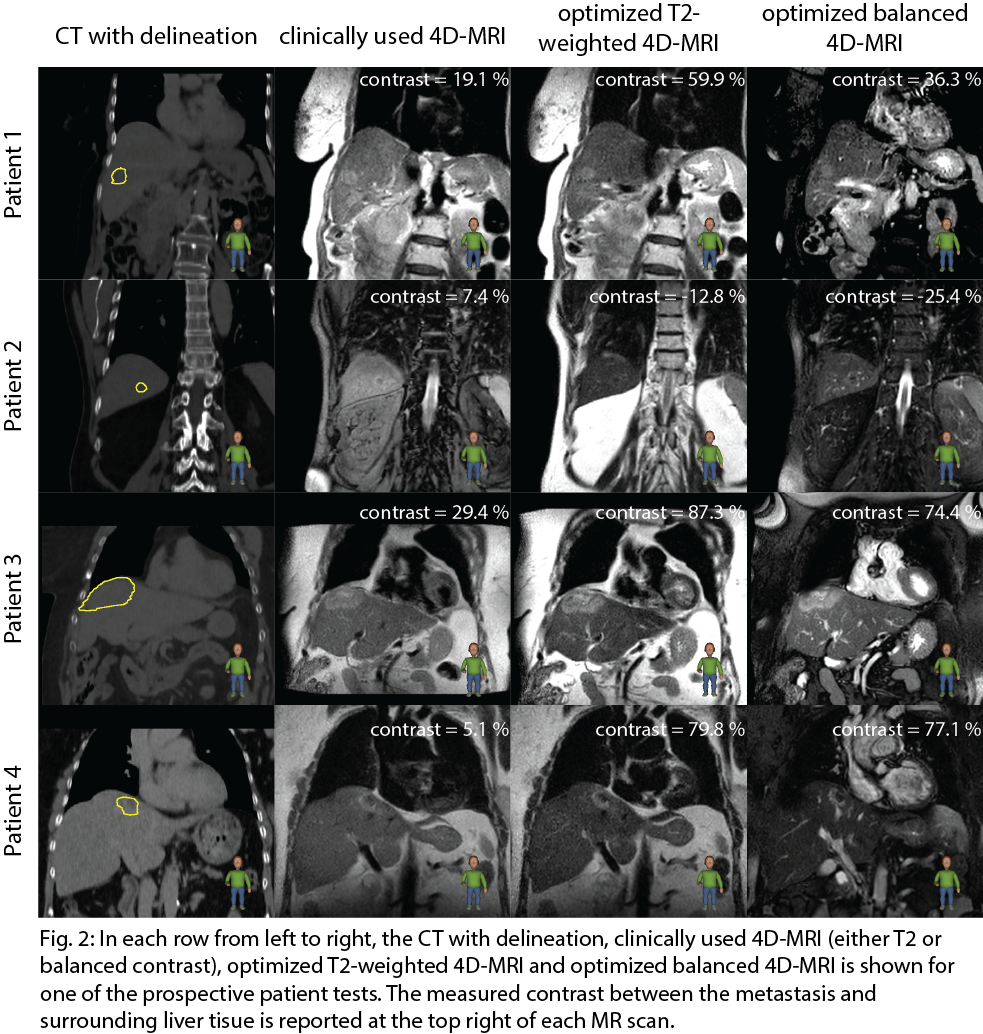
Conclusion
Based on the measured T1 and T2 of metastases and surrounding liver tissue, optimal MRI sequence settings could be calculated. Personalized 4D-MRI sequence settings improved the contrast between a metastasis and surrounding liver tissue in patients prospectively.
References
[1] Weykamp et al., doi:10.3389/fonc.2021.610637