Big data prediction models to select head and neck patients for personalized dose prescription
OC-0755
Abstract
Big data prediction models to select head and neck patients for personalized dose prescription
Authors: Lisanne V. van Dijk1, Sara Ahmed2, Abdallah S R Mohammed2, Kareem Wahid2, Nanna M Sijtsema3, Brandon Gunn2, Adam S Garden2, Johannes A Langendijk4, Clifton D Fuller2
1University Medical Center Groningen; MD Anderson Cancer Center, Radiation Oncology, Groningen, The Netherlands; 2MD Anderson Cancer Center, Radiation Oncology, Houston, USA; 3University Medical Center Groningen, Radiation Oncology, Groningen, The Netherlands; 4University Medical Center Groningn, Radiation Oncology, Groningen, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Current dose de- or escalation trials for
head and neck cancer (HNC) patients generally use tumor staging (including HPV
status) as patient eligibility criteria. Model-based patient selection of low,
intermediate and high-risk patients could improve the effectiveness of
personalized dose strategies. The aim is to develop a robust international overall survival risk-stratification
model based more than 4500 HNC patients.
Material and Methods
The inclusion criteria were HNC patients with
squamous cell carcinomas treated with (chemo)radiation, without prior HN treatment.
Data from 3 different institutes was split into 4 cohorts: a training (n=2241),
independent test (n=786), and 2 external validation cohorts: Cohort 1 (n=1087)
and Cohort 2 (n=497). Data imputation was only used in the training cohort; all
other data was complete.
We constructed: 1) a non-spatial clinical variable model to
establish the outcome risk estimation based on the large-scale data, followed
by 2) optional radiomics (spatial)
component to further prediction improvement.
Training of the Cox regression models was
performed with bootstrapped forward selection. The clinical models were
validated in the independent test and 2 validation cohorts. Subsequently, patients
were stratified into high, intermediate and low risk overall survival
probability based on the predicted 2-year mortality risk; with a priori
thresholds of >25% for high risk and <5% for low risk.
The additional radiomics component was
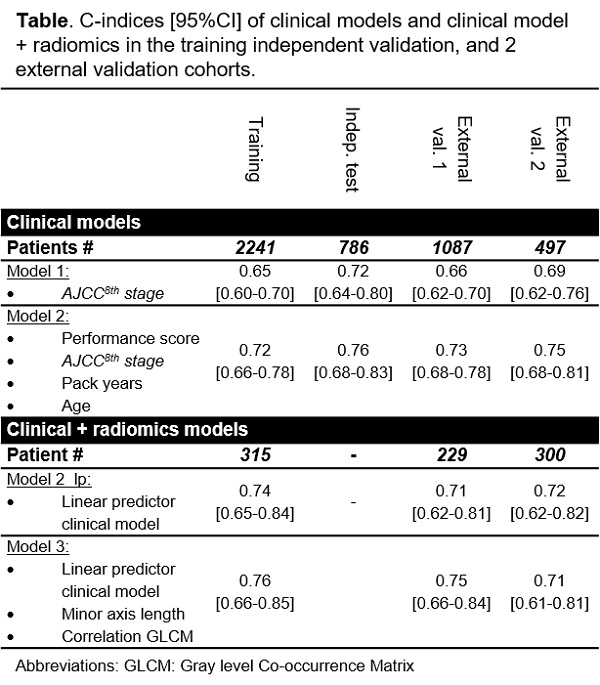
developed in imaging sub cohorts (Table). The selected radiomics predictors
were added to the linear predictor from the final clinical model.
Results
In the multivariable analyses Performance score, AJCC8th stage,
pack years, and Age were selected for the prediction of overall
survival (NB: AJCC8th stage
is based on T and N stage, tumor site and HPV status). Model performance was stable
over different cohorts with c-indices ranging from 0.72-0.76 (Table). The
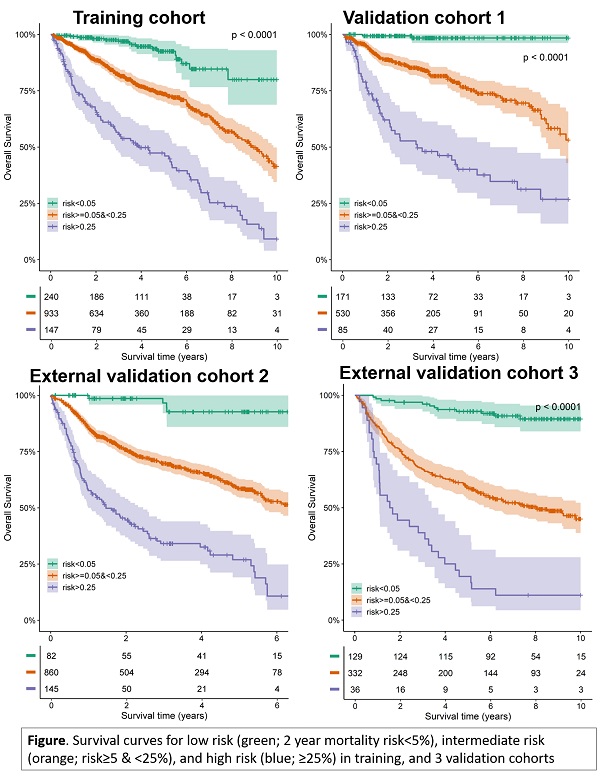
prediction model was highly discriminative for stratifying high, intermediate
and low risk patients (Figure); the cumulative 5-year overall survival ranged
from 92-98% for the low risk group and from 17-46% for the high risk group. The
clinical prediction model outperformed clinical standard-of-care AJCC8th stage prognosis (Table).
In smaller imaging cohorts, the addition of
selected radiomics features to the clinical model’s linear predictor further
improved the performance in training and validation cohort 1(Table).
Conclusion
This international multi-institutional
dataset allowed for the development and validation of a robust overall survival
risk-stratification model. The clinical model showed exceptional distinction
capacity to select low and high-risk patients for potential dose de-escalation
and escalation strategies. Additionally, our right-censoring-aware prediction
model approach provides a framework for robust clinical prediction (i.e.
without refitting), and has simultaneous flexibility to add image features for
further improved risk estimation.