Automated beam angle selection in proton therapy for liver lesions using an AI approach
PD-0730
Abstract
Automated beam angle selection in proton therapy for liver lesions using an AI approach
Authors: Robert Kaderka1, Keng-Chi Liu2, Lawrence Liu3, Reynald Van der Straeten4, Tyng-Luh Liu2, Kuang-Min Lee2, Ethan Tu2, Iain MacEwan3, Daniel Simpson5, James Urbanic3, Chang Chang3
1University of Miami School of Medicine, Radiation Oncology, Miami, USA; 2Taiwan AI Labs, Taiwan AI Labs, Taipei, Taiwan; 3California Protons Cancer Therapy Center, Radiation Oncology, San Diego, USA; 4Varian Medical Systems, Varian Medical Systems, Palo Alto, USA; 5University of California, San Diego, Department of Radiation Oncology and Applied Sciences, La Jolla, USA
Show Affiliations
Hide Affiliations
Purpose or Objective
This
study evaluates the performance of an AI to automate beam angle selection for liver lesions treated with scanned proton beams.
Material and Methods
Forty-nine
patients that received liver treatments between 2017 and 2020 with proton pencil beam scanning were
divided into a training (n=31), validation (n=7), and test
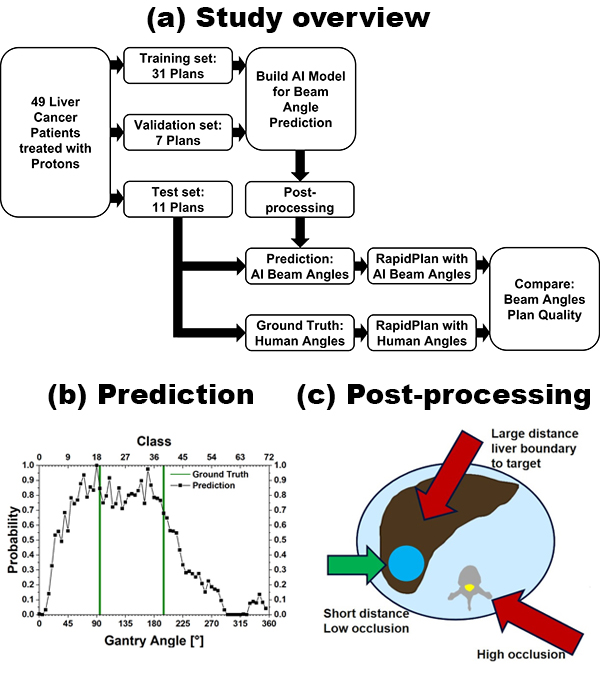
set (n=11) for the AI (Fig. 1a). The AI is based on casting beam-angle selection as a multi-label classification problem. To account for angular boundary discontinuity, the underlying convolution neural network was trained with a novel Circular Earth Mover’s Distance based regularization and multi-label circular-smooth label technique. After training, the network generates a prediction for the probability of each of the 72 classes that represent 5° steps in beam angles (Fig. 1b). The final AI angles are then produced by post-processing with an analytical algorithm that emulates proton planning clinical practice. I.e., the probability is modified for each beam angle based on the distance to the target and organs-at-risk occluding the target (Fig 1c). Performance was evaluated by comparing AI beam angles with the ground truth,
i.e., angles selected by human planners. Additionally, knowledge-based treatment planning was employed to automatically create treatment plans and assess the impact of beam angle choice on several dosimetric parameters.
Results
For
8 of the 11 cases in the test set, AI-selected beam angles agreed with those
determined by human planners to within 20 degrees (median angle difference =
10°; mean = 18.6°). Moreover, out of the total 22 beam angles predicted by the
model, 15 (68%) were within 10 degrees of the human-selected angles (see table).
| Case No. | Human Beam Angles [°] | Predicted Beam Angles [°] | AI Angles after post-Processing [°] |
| 39 | 75, 130 | 0, 70 | 0, 70 |
| 40 | 0, 90 | 90, 170 | 90, 170 |
| 41 | 20, 105 | 40, 110 | 40, 110 |
| 42 | 45, 115, 180 | 40, 70, 110 | 40, 70, 170 |
| 43 | 90, 115 | 40, 125 | 90, 125 |
| 44 | 90, 180 | 90, 170 | 90, 170 |
| 45 | 45, 345 | 90, 355 | 90, 355 |
| 46 | 0 | 0, 90 | 0, 40 |
| 47 | 75, 190 | 40, 110 | 110, 160 |
| 48 | 80, 340 | 90, 355 | 90, 355 |
| 49 | 85, 175 | 90, 160 | 90, 160 |
| Mean Difference |
| 26.2 | 18.6 |
| Median Difference |
| 12.5 | 10.0 |
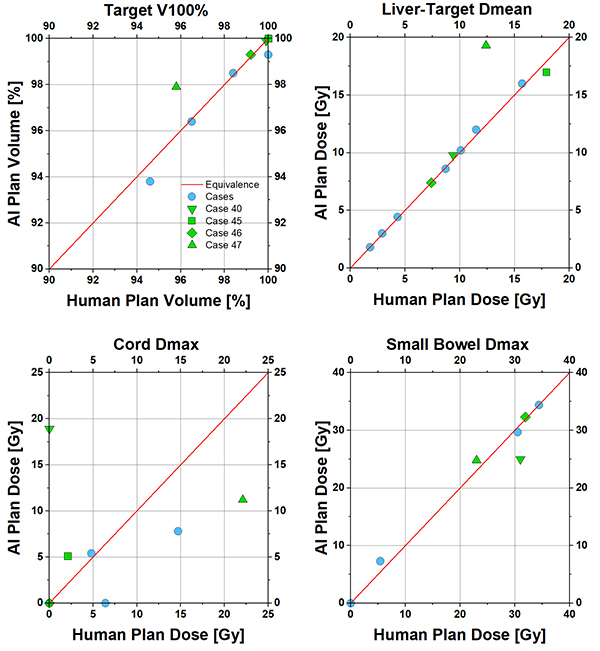
The high correlation of human and AI predicted beam angles resulted in comparable plans in terms of dosimetric parameters (Fig 2). Select cases showed significant angle differences, dosimetric differences and their
implications to overall plan quality will be discussed in terms of plan robustness,
distal stoppage, and optimization settings.
Conclusion
The AI proposed in this study was able to
automatically select beam
angles for proton liver treatment planning that were close to the
human-selected ground truth. Treatment plans using these AI-selected angles achieved comparable dose
distributions to those using human-selected angles. In conjunction with
knowledge-based optimization methods this could be a tool to efficiently automate proton treatment planning in liver lesions.