Rectal-spacer hyaluronic acid plus high-dose-rate prostate brachytherapy boost: long-term outcomes
MO-0300
Abstract
Rectal-spacer hyaluronic acid plus high-dose-rate prostate brachytherapy boost: long-term outcomes
Authors: Alai Goñi Ramirez1, Belen De Paula Carranza1, Eva Saenz de Urturi Albisu1, Maria Pagola Divasson1, Mikel Egiguren Bastida1, Nuria Bulto Boqué1, Arancha Ayete Andreu1, David Ignacio Ortiz de Urbina Ugarte1, Vicent Pastor Sanchis2, Albert Bartres Salido2, Noelia Suarez2, Melanie Erzilbengoa2, Jesus Rosa Nieto1
1Fundación Onkologikoa - UGC Oncología Gipuzkoa, Radiation Oncology, San Sebastian, Spain; 2Fundación Onkologikoa - UGC Oncología Gipuzkoa, Medical Physics, San Sebastian, Spain
Show Affiliations
Hide Affiliations
Purpose or Objective
To determinate and evaluate long-term survival, dosimetric
and gastrointestinal (GI) toxicity outcomes after rectal-spacer hyaluronic acid
(HA) injection during high-dose-rate brachytherapy (HDR-BT) boost in treatment
of high-risk prostate cancer patients.
Material and Methods
Between january-2009 and december-2017, 234 patients with high-risk
prostate cancer were treated in our institution with CT-based iridium192 15Gy HDR-BT
boost and external beam radiotherapy (EBRT) combination schema. All patients
received transrectal ultrasound-guided HA rectal-spacer injection in Denonvilliers
fascia during HDR-BT procedure. After HDR-BT boost, all patients received EBRT
46Gy in 23 fractions of pelvic irradiation encompassing the prostate and
seminal vesicles. Those patients with less than 3-year follow-up and less than
3 post treatment prostate-specific antigen (PSA) were excluded. Survival, dosimetric
and GI toxicity outcomes were evaluated. Toxicity was evaluated using Common
Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Results
About 202 from 234 patients satisfied inclusion
criteria. Median follow-up was 8 years. Median age was 71 years (range: 49-83) and
85.1% of patients received androgen deprivation therapy (ADT), 55% long-term
(36 months). Median PSA was 11ng/m and 67.7% of patients was Gleason 7 (4+3) or
superior. T3 or superior clinical stage was 70.8%. All patients received at
least 2cc of HA between prostate and rectum. Freedom from biochemical failure
(FFBF) defined by Phoenix-criteria was 88.1% at 5 years and 76.1% at 10 years, respectively.
Metastasis free survival (MFS) and Overall Survival (OS) was 95.9% and 92.7% at
5 years and 89.97% and 76.7% at 10 years, respectively. For HDR-BT boost target, median D90 was 109Gy
and v100 98%. Analyzing rectum dosimetry, 2cc dose median was 8Gy and
max dose median at 0.1cc was 10Gy. Combination of 15Gy HDR-BT and EBRT had a
median BED (α/β=3) in rectum of 104Gy with a median equivalent
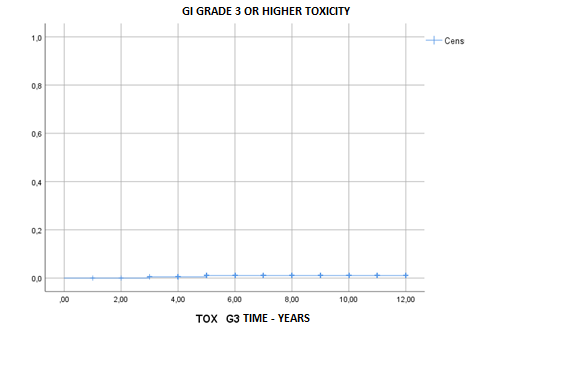
dose of 62Gy. GI grade 2 late toxicity was 0.7% at 5 years and 1.1% at 10
years, respectively, and GI grade 3 or higher late toxicity
was 0.1% for both 5 and 10 years.
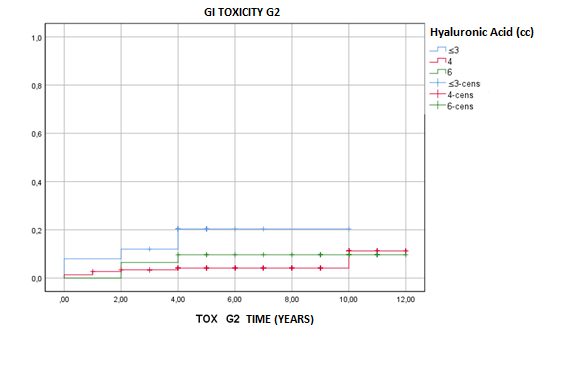
Subdivided according to injected HA quantity, GI grade 2 late toxicity was
statistically unfavorable for those patients who were injected with 3cc or less
HA. At 5 and 10 years, GI grade 2 late toxicity
was 79.6% and 79.6% for 3cc injected, 95.8% and 88.7% for
4cc injected and 90.3% and 90.3% for 6cc or more injected, respectively. No
significant difference was found in GI grade 3 or higher late GI toxicity
between HA injection groups.
Conclusion
HA rectal-spacer injection during HDR-BT boost was
well tolerated and provided excellent long-term toxicity and survival outcomes
in patients with high-risk prostate cancer in combination with EBRT. Late grade
2 GI toxicity was statistically unfavorable for those patients with less
quantity of HA injected. No significant difference was found in GI grade 3 or
higher late GI toxicity.