Simultaneous ThermoBrachytherapy can improve OAR sparing in prostate HDR Brachytherapy
Ioannis Androulakis,
The Netherlands
OC-0276
Abstract
Simultaneous ThermoBrachytherapy can improve OAR sparing in prostate HDR Brachytherapy
Authors: Ioannis Androulakis1, Rob M.C. Mestrom2, Inger-Karine K. Kolkman-Deurloo1, Miranda E.M.C. Christianen1, Gerard C. van Rhoon1
1Erasmus MC, Radiotherapy, Rotterdam, The Netherlands; 2TU Eindhoven, Electrical Engineering, Eindhoven, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Thermotherapy is a known
sensitizer to radiation (Horsman MR, Overgaard
J.; Clin. Oncol.; 2007) and is known to
lower the α/β of tumors (Datta NR, Bodis S.; Radiother. Oncol.; 2019). The thermal enhancement
ratio (TER) of the radiation dose is, however, known to be dependent on the time
interval between the radiation and thermal dose delivery, with the highest TER
for simultaneous application of the two modalities. Simultaneous
ThermoBrachytherapy (STBT) is defined as HDR-BT with simultaneous interstitial thermotherapy
assuming the same equivalent dose (EQD) to the target by sensitization and
lower physical BT dose (Androulakis
I, et al.; Int. J. Hyperth. 2021). In
this study we investigated what OAR dose reduction can be expected when HDR-BT
only is replaced by STBT in low and intermediate risk prostate cancer (PCa).
Material and Methods
The
effect of the combined TBT treatment was quantified using the temperature
dependent LQ model (Van Leeuwen CM, et al.; Int. J. Hyperth. 2017). We compared the physical HDR-BT fraction dose
delivered to 10 previously irradiated PCa patients with a STBT treatment. In
the original treatment consisting of 2 fractions, the prescribed dose was Dp = 13.5
Gy per fraction. For the TBT simulations we assumed 85% of the original HDR-BT dose
and added an EQD-optimized simultaneous thermal dose fraction of 1h with a
maximum temperature constraint of 47 °C, using the same dose objectives and
constraints (Fig. 1). For all tissues we assumed an α/β = 3 Gy. For the target, the temperature dependence of α (α43/α37)
and β (β43/β37) was based on PC-3 and DU-145 PCa cell line data
(Pajonk F, et al.; Cancer Res.; 2005). As there is limited thermoradiotherapeutic
data available on healthy tissues, we investigated α43/α37
and β43/β37 ranging from 1 to the value assigned to PCa . We
evaluated the target coverage (V100%), as well as the urethra D0.1cc,
rectum D1cc, and bladder D1cc, accounting for the
variability due to different α43/α37
and β43/β37 values. Differences in dose–volume metrics were
evaluated for statistical significance using a paired sampled Wilcoxon signed
rank test with p < 0.001.
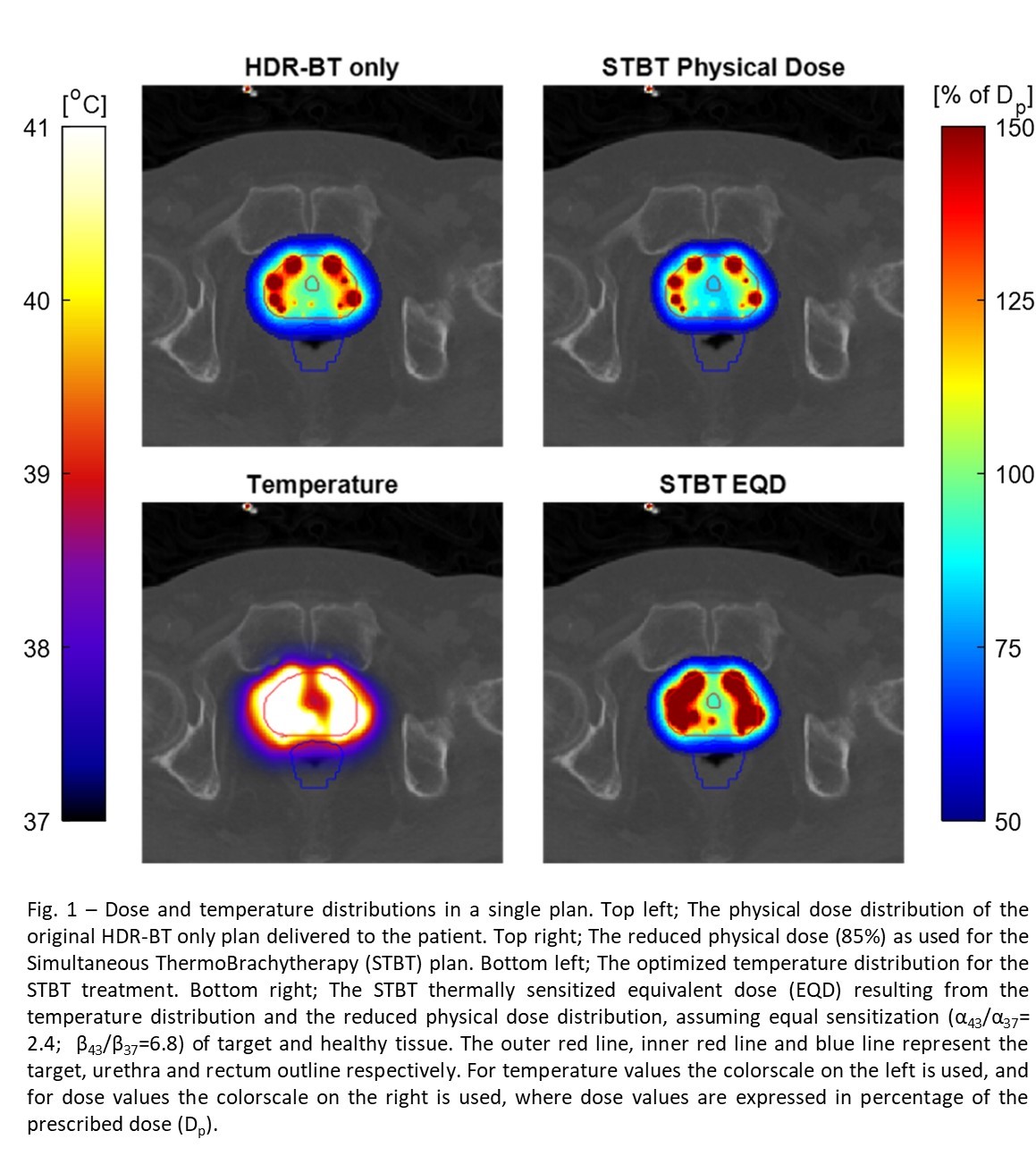
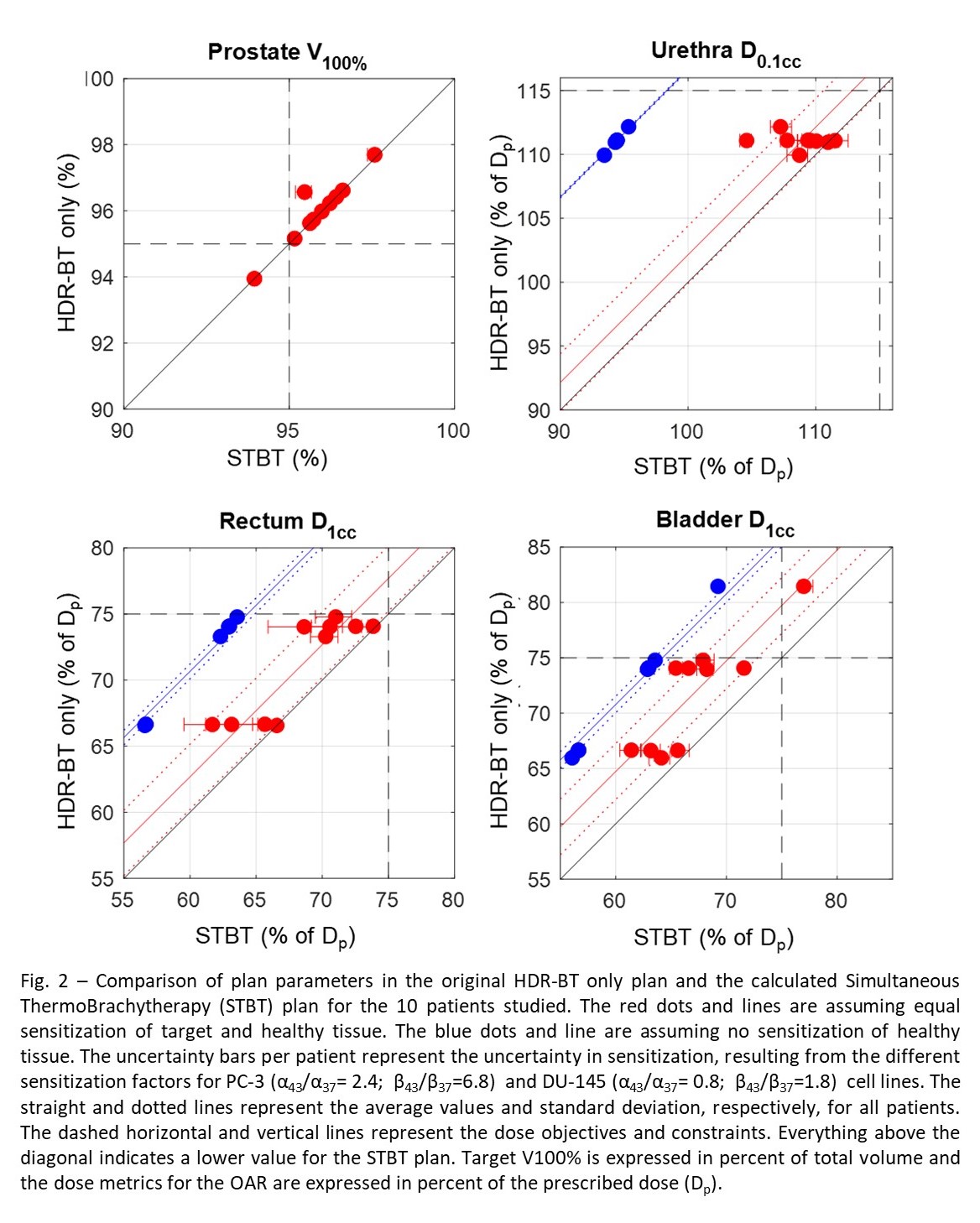
Results
The target objective was
reached, with no significant difference in V100% between the HDR-BT
only and STBT plan (0.1%±0.3%). For the OAR, the dose reduction was significant
in all cases. For the scenario of equal sensitization of healthy and PCa
tissue, the reductions in the urethra D0.1cc , rectum D1cc,
and bladder D1cc, were 2.2%±2.3%, 2.7%±2.5%, and 4.7%±2.5%,
respectively (Fig. 2). For the scenario of no sensitization of healthy tissue,
the reductions were 16.7%±0.1%, 10.7%±0.6%, and 10.8%±0.7%, respectively (Fig.
2).
Conclusion
Our calculations indicate that
STBT has the potential to reach the same target coverage with a significantly
lower dose to the OAR in monotherapy for low and intermediate risk PCa. For a final conclusion including
clinical relevance, more information on the temperature dependence of α and β
for normal tissue is needed.