Incorporating control of contiguous high-dose volumes in automated optimization for prostate BT
Anton Bouter,
The Netherlands
OC-0275
Abstract
Incorporating control of contiguous high-dose volumes in automated optimization for prostate BT
Authors: Joost L.P. Commandeur1, Anton Bouter1, Leah R.M. Dickhoff2, Danique L.J. Barten3, Henrike Westerveld3, Bradley R. Pieters3, Tanja Alderliesten2, Peter A.N. Bosman1
1Centrum Wiskunde & Informatica, Life Sciences and Health, Amsterdam, The Netherlands; 2Leiden University Medical Center, Radiation Oncology, Leiden, The Netherlands; 3Amsterdam UMC University of Amsterdam, Radiation Oncology, Amsterdam, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
In 2020, ‘BRachytherapy via artificially
Intelligent GOMEA-Heuristic based Treatment planning’ (BRIGHT) for
prostate HDR BT was clinically introduced. BRIGHT is a bi-objective treatment
planning method that finds a set of high-quality, patient-specific treatment
plans (TPs) with different trade-offs between clinical target coverage and organ
sparing, by directly optimizing the dose volume indices (DVIs) in the clinical
protocol. However, in the clinic, manual adjustments of BRIGHT TPs are still done
to meet additional patient specific aims. Particularly, this includes minimization
of contiguous high-dose volumes, i.e., hotspots (HSs). We therefore aim to incorporate
control of HS volumes in BRIGHT, while minimally impacting obtainable DVI
values.
Material and Methods
We augment BRIGHT with a third objective
to minimize HSs. For this, we define an HS as ‘a contiguous volume of >0.1 mL
outside catheters receiving >300% in target volumes: prostate and seminal
vesicles, or >200% in normal-tissue around target volumes of the prescribed
dose’. We tailored a graph-based method, which uses a connected component
algorithm (Afforest), to determine HSs. The graph consists of dose calculation
points (DCPs) as nodes and edges between close (≤0.5 mm) neighbouring DCPs. DCPs are
randomly sampled locations where the dose is calculated (to compute the DVIs).
The third objective in tri-objective
BRIGHT is the sum of HS volumes (metric 1). For comparison, we also consider as
third objective a more efficiently computable metric, which however ignores
whether the high-dose volume is contiguous: the sum of V300% of the
target volumes and V200% of normal tissue (metric 2).
We compare bi-objective BRIGHT with both
tri-objective BRIGHT versions on a data set of 11 prostate cancer patients by
retrospectively planning single-dose HDR BT with 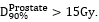
Results
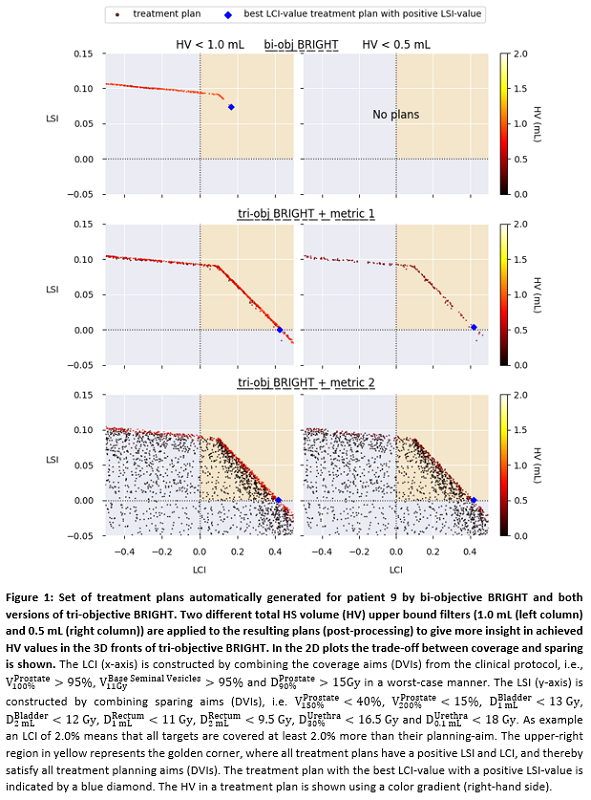
Figure 1 shows for patient 9 that,
both tri-objective BRIGHT versions result in a clear improvement in control of HSs;
TPs with HSs ≤0.5 mL are only
found using the tri-objective versions. Due to the nature of metric 2 and using
a 2D plot for a 3D front, the trade-off between existing DVIs and metric 2
culminates in a larger covered area with TPs with low HS volumes.
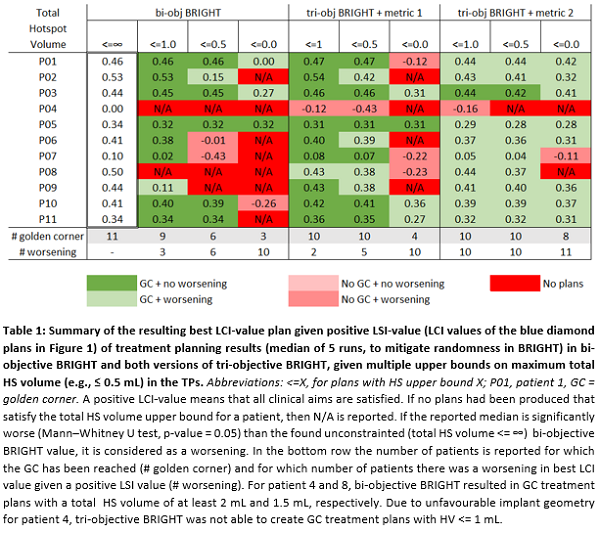
Table 1 shows that when using
metric 1, for 10 out of 11 patients, total HS volume could be reduced to ≤0.5 mL while satisfying the clinical
protocol, versus 6 out of 11 patients for bi-objective BRIGHT. Adding metric 1
does not result in worsening of DVIs. Adding metric 2 does cause slight worsening
of DVIs but results in more plans satisfying the clinical protocol without HSs.
Currently, using metric 1 and 2
takes 1800s and 600s, respectively. Metric 1 needs further optimization to
definitively assess runtime impact.
Conclusion
We successfully adapted BRIGHT to
reduce HSs without compromising obtainable DVI values for most patients, by
explicitly computing HSs and minimizing their volume through a third objective.
This could potentially render manual HS adjustments redundant.