External validation of an NTCP model for patient reported xerostomia in a multicenter cohort
Grete May Engeseth,
Norway
MO-0882
Abstract
External validation of an NTCP model for patient reported xerostomia in a multicenter cohort
Authors: Grete May Engeseth1,2, Cecilie Delphin Amdal3, Liv Bolstad Hysing1,4, Åse Bratland3, Ludvig Paul Muren5, Jannicke Nøkling Moi1, Kristin Søvde6, Morten Evensen3, Solveig Undheim Thomassen3, Marianne Brydøy1
1Haukeland University Hospital, Department of Oncology and Medical Physics, Bergen, Norway; 2The University of Bergen, Department of Clinical Science, Bergen, Norway; 3Oslo University Hospital, Department of Oncology, Oslo, Norway; 4The University of Bergen, Department of Physics and Technology, Bergen, Norway; 5Aarhus University Hospital, Danish Center for Particle Therapy, Aarhus, Denmark; 6Haukeland University Hospital, Department of Oncology and Medial Physics, Bergen, Norway
Show Affiliations
Hide Affiliations
Purpose or Objective
Radiation
induced xerostomia following radiotherapy in the head and neck region is a
troublesome late effect affecting patients’ quality of life. Normal Tissue
Complication Probability (NTCP) models for predicting xerostomia have been
developed, but external validation is needed in order to determine validity and
generalizability in patient cohorts not used for model development. Here we
present results from the external validation of an NTCP model for predicting
patient reported moderate to severe xerostomia in a multicenter cohort.
Material and Methods
A total of 167 patients
had completed the EORTC HN35 questionnaire at baseline and 6 months post-radiotherapy
where mouth dryness was scored as “not at all” (Xer1), “a little” (Xer2), “quite
a bit” (Xer3) and “very much” (Xer4). The predictors in the original NTCP model
were the mean dose (Dmean) to the contralateral parotid gland,
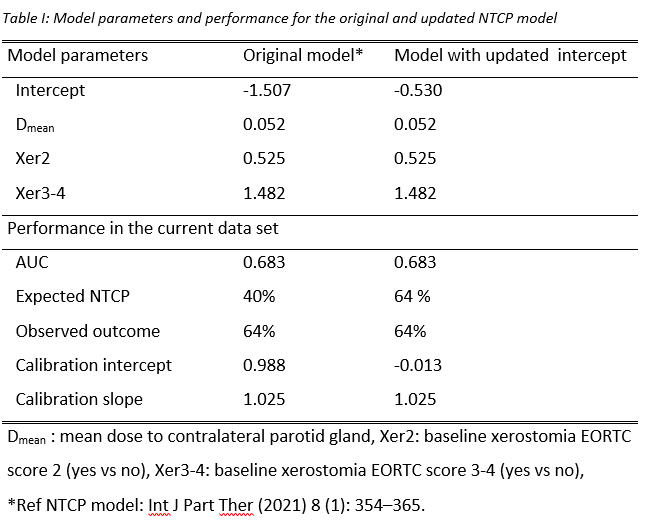
baseline Xer2 and baseline Xer3-4, respectively (Table I). The model endpoint
was moderate to severe xerostomia (i.e. Xer3-4) six months after completion of
treatment. For model validation a closed testing procedure was applied.
Successively, recalibration in the large (i.e. model intercept update),
recalibration and model revision were performed, whereupon predictive
performance was tested for significant improvements compared to original model
in each step. Bootstrapping (n = 1000) was perform to investigate the robustness
of the procedure.
Results
At baseline, Xer1
was reported in 79 patients (47%), Xer2 in 53 patients (32%) and Xer3-4 in 35 patients
(21%). Six months post-treatment the prevalence rate of Xer3-4 was 64%. The median
(IQR) Dmean to the contralateral gland were 11.5 (5.5-17.1) Gy and 15.0
(6.3-22.4) Gy for those with Xer1-2 vs. Xer3-4 (p = 0.04). In the bootstrap closed
testing procedure, calibration in the large was selected 889 times, model
revision 82 times, recalibration of the model 28 times and the original model
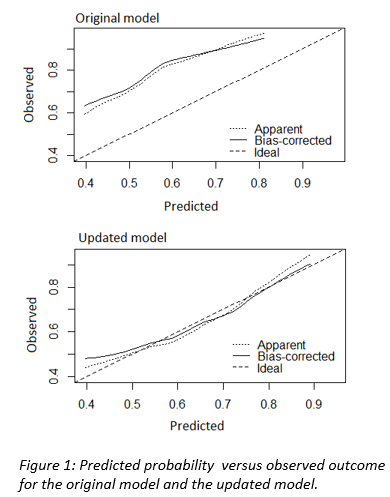
one time. The closed testing showed that by refitting the model intercept, the
model matched the current patient cohort well with improved calibration
compared to the original model (Table I/ Figure 1). For patients with EORTC score 1 vs. 2-4 at
baseline, median (IQR) estimated risk of Xer3-4 were 0.50 (0.44-0.80) and 0.76
(0.61-0.79). The corresponding observed rates of Xer3-4 in the two groups were 52% and 75%.
Conclusion
Six months after
treatment the majority of patients reported moderate to severe xerostomia. The closed testing procedure resulted
in an intercept update of the original model. The original model underestimated
the risk of moderate to severe xerostomia, whereas the validated model was
well-calibrated and had good discriminative ability.