Proton Beam Therapy for Central Nervous System tumours: outcomes from the Proton Overseas Programme
Simona Gaito,
United Kingdom
MO-0883
Abstract
Proton Beam Therapy for Central Nervous System tumours: outcomes from the Proton Overseas Programme
Authors: Simona Gaito1,2, Eunji Hwang3,4, Anna France1, Gillian Whitfield3,2, Shermaine Pan5, Gareth Price2, Marianne Aznar2, Adrian Crellin6, Daniel Indelicato7, Ed Smith3,1,2
1The Christie NHS Foundation Trust, Proton Clinical Outcomes Unit, Manchester, United Kingdom; 2University of Manchester, Manchester Cancer Research Centre, Manchester, United Kingdom; 3The Christie NHS Foundation Trust, The Christie Proton Beam Therapy Centre, Manchester, United Kingdom; 4Institute of Medical Physics, School of Physics, University of Sidney, Australia; 5The Christie NHS Foundation Trust, The Christie Proton Beam Therapy centre, Manchester, United Kingdom; 6NHS England, National Clinical Lead Proton Beam Therapy, Manchester, United Kingdom; 7University of Florida, Department of Radiation Oncology, Jacksonville, USA
Show Affiliations
Hide Affiliations
Purpose or Objective
In 2008, the UK National Health
Service (NHS) started the Proton Overseas Programme (POP), to provide access for
Proton Beam Therapy (PBT) abroad for selected tumour diagnoses, whilst two
national centres were being planned. This work reports the moderate-severe
toxicities and their incidence in the patient group treated for Central Nervous
System (CNS) malignancies.
Material and Methods
Since the start of the NHS POP
Programme, an agreement between NHS England and UK referring centres ensured
outcomes data collection. Follow-up correspondence has been stored in patient
files in a national database and curated by the Proton Clinical Outcomes Unit,
established in 2018.
Clinical and treatment-related data
were extracted from the central patient database. The POP patient cohort was
divided into CNS and extracranial diseases. Spinal and skull base (BoS)
chordoma and chondrosarcoma were grouped with CNS diseases. Grade (G) ≥3 late toxicities (LT), as per
CTCAE (Common Terminology Criteria for Adverse Events) v 4.0 definition,
occurring later than 90 days since completion of treatment, were recorded. Where toxicity could not be graded
accurately, individualised workbooks were sent to referring centres for
clarification. The follow up time is calculated from end of PBT treatment to death
or last follow up.
Results
Between 2008 and September 2020,
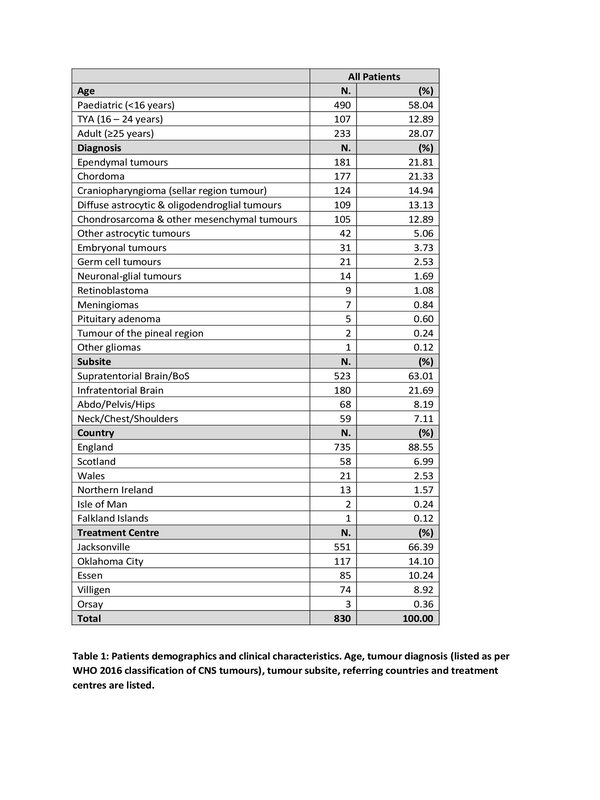
830 patients were treated within the POP for CNS malignancies. Their
demographics and clinical characteristics are listed in Table 1. After a median
follow up of 2.65 years (range 0.03 - 11.59) the overall survival for the whole
cohort was 91.33%, and the local control was 75.42%. Of note, the local control
was unknown in 12.29% of the patients, and 12.29% experienced disease
progression. Toxicity analysis (Table 2) was carried out on 760 patients, with
patients excluded due to short follow-up (<90 days) and/or inadequate
toxicity data available. Median follow up in this population is 2.8 years (0.26
- 11.59), and median radiotherapy prescription dose 54 GyRBE (34.8-79.2).
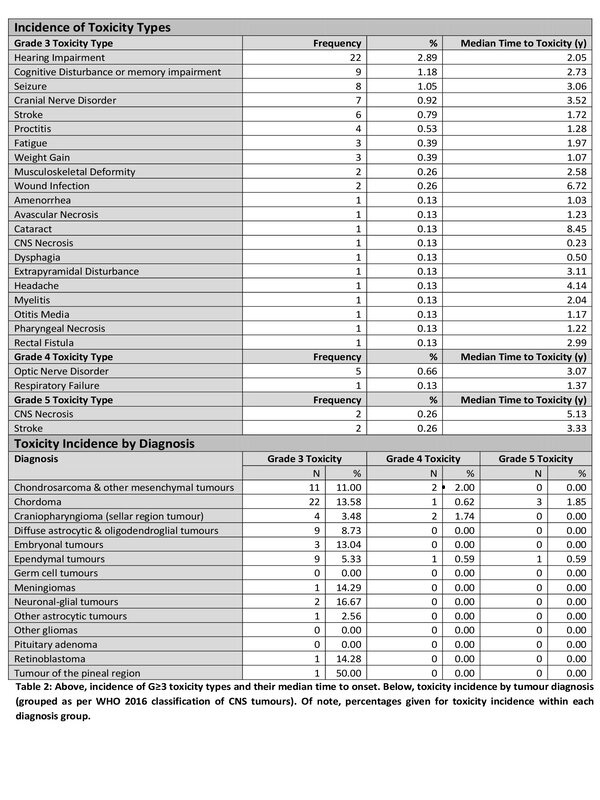
In total, 69 patients (9.1%)
experienced G≥3 LT, with 18 (2.4%) experiencing
more than one toxicity. Of note, G≥3 LT are more common in those tumour groups (such as Chordomas and
Chondrosarcomas) receiving dose-escalated treatments, and in those (such as
Craniopharyngiomas and Ependymoma) commonly located in close proximity to
critical organs at risk and background of multiple surgical procedures.
Conclusion
The results of this study
indicate safety of PBT for CNS tumours, with predominantly passive scattering.
Clinical outcomes from this cohort will be compared with the newest Pencil Beam
Scanning technology, in the UK NHS National PBT service. Baseline toxicity
assessment is essential for the correct interpretation of radiotherapy
toxicities and longer follow up is needed to evaluate endpoints, such as
secondary malignancies, which have a long latency period.