Durvalumab increases the risk of radiation pneumonitis in non-small-cell lung cancer patients
Rutger H. Stoffers,
The Netherlands
MO-0387
Abstract
Durvalumab increases the risk of radiation pneumonitis in non-small-cell lung cancer patients
Authors: Rutger Stoffers1, Anne G.H. Niezink1, Olga Chouvalova1, Annija H.D. van der Leest1, Jan F. Ubbels1, Marleen Woltman-van Iersel1, Johannes A. Langendijk1, Robin Wijsman1
1University Medical Center Groningen, Radiation Oncology, Groningen, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
The addition of adjuvant durvalumab to
chemoradiotherapy (CRT) in locally advanced
NSCLC resulted in a significant improvement of OS and PFS. Several single centre
studies reported on an increase in adverse events, in particular in radiation pneumonitis
(RP). However, large cohort studies presenting real-world data are lacking. The
aim of this study was to investigate the incidence of RP after CRT with adjuvant
durvalumab immunotherapy in patients with locally advanced NSCLC.
Material and Methods
The study population was composed of all patients
with NSCLC that completed CRT with curative intent between February 2013 and
October 2020. From 2018 on, adjuvant durvalumab immunotherapy was administered
in patients with locally advanced stage (II-III) disease, a WHO performance of 0-1
and without disease progression after completion of CRT. Patient and tumour
characteristics, dosimetric RT data, chemotherapy and immunotherapy data together
with RP data (CTCAE v4.0, scored up to 9 months after CRT), were prospectively
collected as part of our standard follow up program.
Results
A total of 318 patients
were enrolled, of which 97 (30.5%) received adjuvant durvalumab. Baseline patient
and tumour characteristics did not differ between the two groups. Patients in
the durvalumab group received proton therapy more often. A significant increase
in the incidence of grade ≥2 RP in patients receiving durvalumab compared to
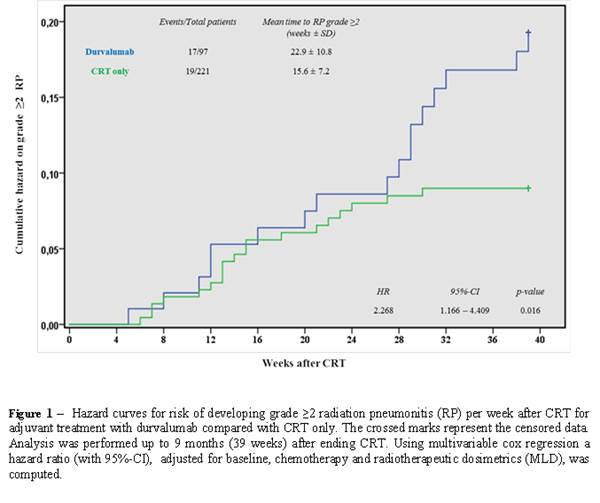
CRT only was observed (17.5% vs. 8.6%; HR: 2.27, 95%CI: 1.17-4.41, p=0.016). In
the first six months after ending CRT, both groups had equal cumulative RP
incidences. Interestingly, after these six months, patients receiving adjuvant
durvalumab developed RP more frequently compared to patients treated with only
CRT (Figure 1). In multivariable logistic regression analysis, adjuvant
treatment with durvalumab was significantly associated with a higher incidence
of grade ≥2 RP (HR 2.57, 95%CI: 1.24-5.33, p=0.011). Also, the risk of RP
increased with higher MLD (HR 1.16, 95%CI: 1.04-1.29, p=0.009). Treatment with
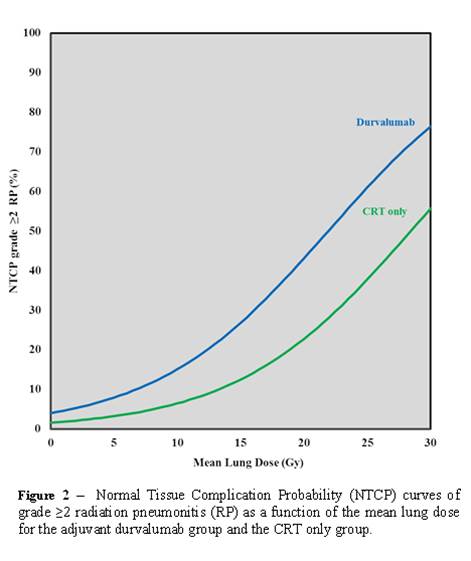
adjuvant durvalumab results in an additional probability on developing grade ≥2
RP, especially when the MLD is high (Figure 2). The 2-years OS
in the durvalumab group was 80.8% compared to 57.5% after CRT only (p=0.027).
Patients treated with durvalumab had a decreased hazard on death in the first 2
years after completing CRT (HR 0.42, 95%CI: 0.18–0.95, p=0.038).
Conclusion
Treatment with adjuvant durvalumab significantly
increased the incidence of grade ≥2 RP in this cohort of real-world NSCLC
patients. Furthermore, the time patients are prone to develop grade ≥2 RP after
ending CRT was longer in patients receiving adjuvant durvalumab compared to
patients treated with CRT only.