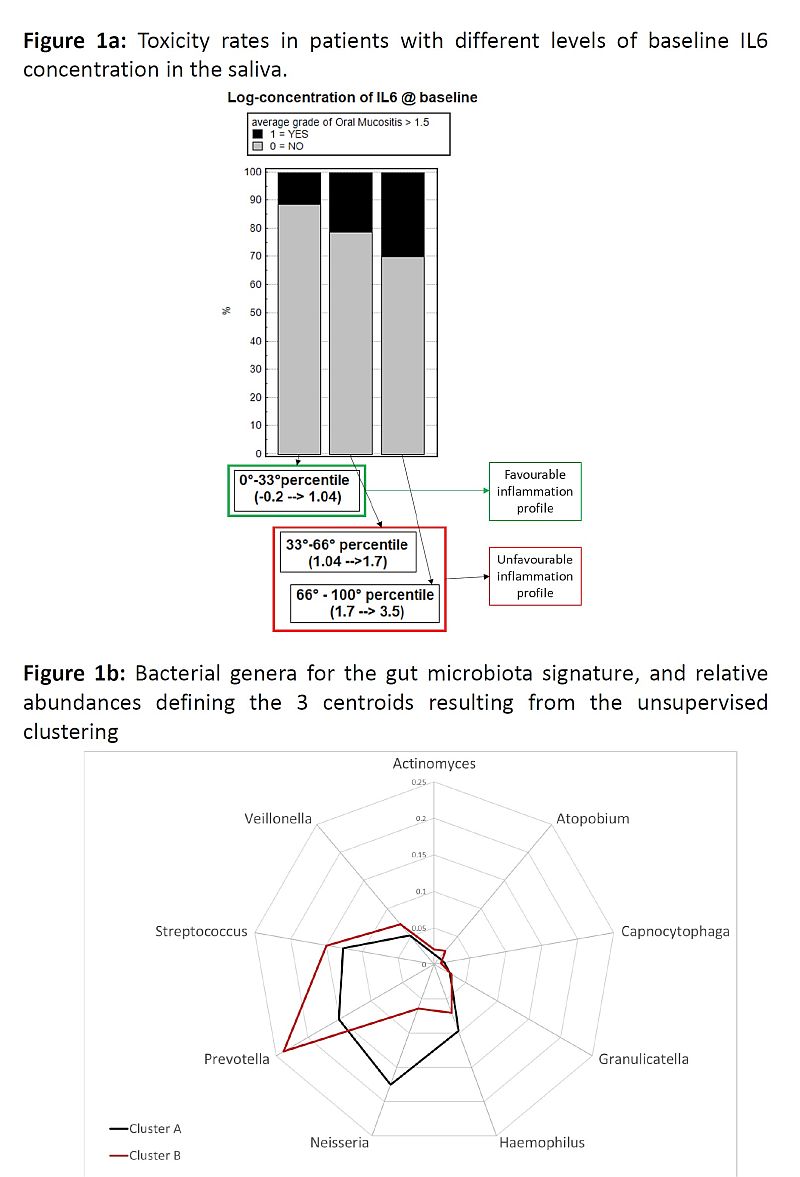
Toxicity was scored in 22/114
pts.
The baseline concentration of IL6 was significantly associated with acute tox:
OR=1.8 (continuous log-scale, p=0.05). Fig1a presents results when stratifying
at 33°-66° percentiles OR=1.8 for each step (p=0.04). We defined a favourable
IL6 profile if IL6 in the lowest 10° percentile (logIL6 (ng/ml) <0.7), 15.4
vs 36.8% aOM in favourable vs unfavourable IL6.
MB clustered in 2 groups at the Genus level, with 9
genera included in the centroid signature (Fig1b). With Haemophilus, Neisseria,
Prevotella and Streptococcus mostly driving the pts grouping. Pts in cluster B
had a significantly higher probability of aOM (unfavourable MB) compared to pts
in cluster A (favourable MB): tox rates were 22.7 vs 14.6%, OR=1.7 (p=0.05).
MB clustering was confirmed in the validation
cohort: tox rates 19 vs 32% in unfavourable vs favourable MB (without any
change in centroids for clustering).
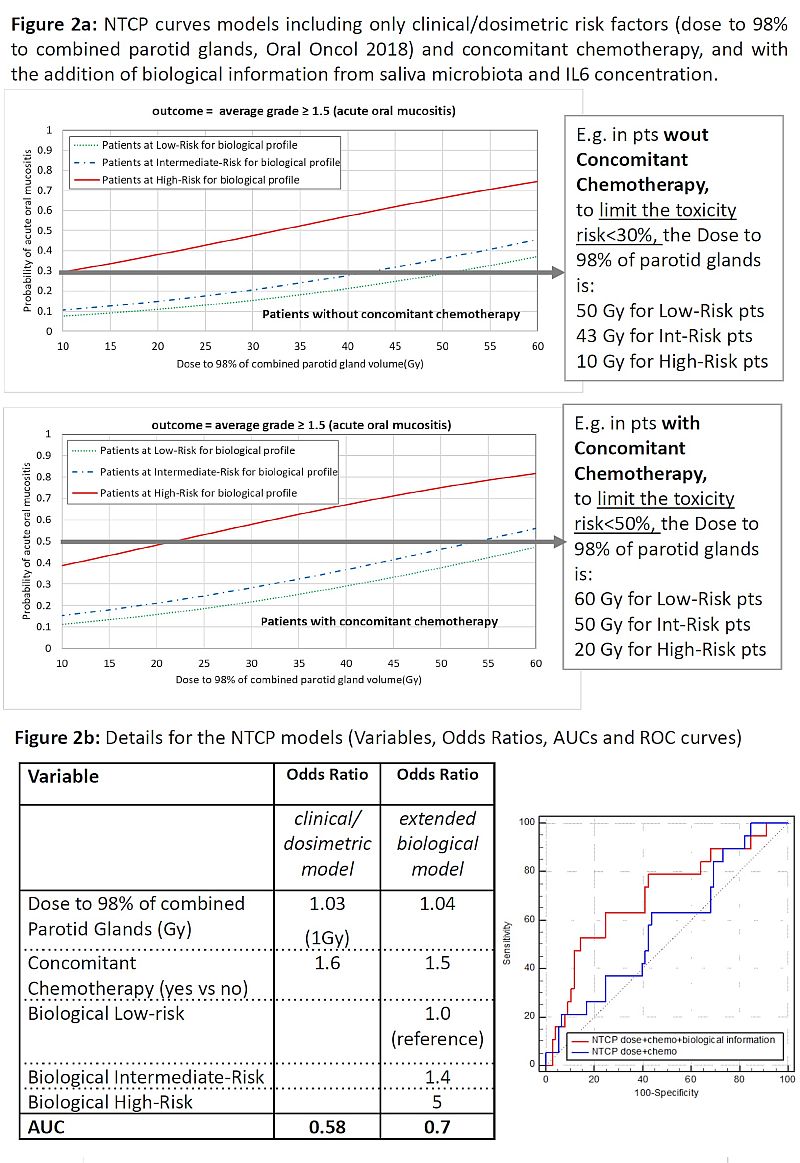
To join
information from MB and inflammation marker, we classified pts at low-risk (LR)
of tox if they had “favourable MB AND IL6 profile”, at intermediate-risk (IR)
if “favourable MB OR IL6 profile”, at high-risk (HR) if “unfavourable MB AND IL6
profile”. Observed toxicity rates in LR/IR/HR were 12.5/16.7/41.2%
(p=0.04).
We obtained different tolerance doses for different
risk classes when including “biological” stratification into a NTCP model
(Fig.2).