MR-guided SBRT of infra-diaphragmatic metastases – the first 100 patients included in the SOFT trial
MO-0714
Abstract
MR-guided SBRT of infra-diaphragmatic metastases – the first 100 patients included in the SOFT trial
Authors: Mette Felter1, Pia Krause Møller2, Mirjana Josipovic3, Susanne Nørring Bekke4, Uffe Bernchou2, Eva Serup-Hansen5, Parag Parikh6, Kim Joshua7, Poul Geertsen4, Claus P. Behrens4, Ivan R Vogelius3, Mette Pøhl3, Tine Schytte2, Gitte Persson4
1Copenhagen University Hospital - Herlev and Gentofte, , Department of Oncology, Herlev, Denmark; 2Odense University Hospital, Department of Oncology, Odense, Denmark; 3Copenhagen University Hospital - Rigshospitalet, Department of Oncology, Copenhagen, Denmark; 4Copenhagen University Hospital - Herlev and Gentofte, Department of Oncology, Herlev, Denmark; 5Copenhagen University Hospital - Herlev and Gentofte, Department of oncology, Herlev, Denmark; 6Henry Ford Hospital, Department of Oncology, Detroit, USA; 7Henry Ford Hospital, Departement of Oncology, Detroit, USA
Show Affiliations
Hide Affiliations
Purpose or Objective
Reports from
recent years suggest a more aggressive approach for oligometastatic disease (OMD)
may prolong overall survival.SOFT is a multicenter,
phase II trial investigating the safety and efficacy of MR-guided stereotactic
body radiotherapy (SBRT) of infra-diaphragmatic soft tissue metastases in
patients with OMD (clinicaltrials.gov ID NCT04407897). The primary endpoint is
severe toxicity rate within the first year. We report the baseline
characteristics and target doses for the first 100 patients included in the trial.
Material and Methods
Patients with OMD
(≤ 5 metastases) or oligo-progressive disease (OPD) (≤ 3
metastases) are eligible for inclusion at four centers. Our strategy is to
pursue safe SBRT for all metastatic sites by heterogeneous dose prescription,
where organs at risk (OAR) constraints are prioritized over target coverage. Doses
of 45 Gy/3 fractions (F), 50 Gy/5 F or 60 Gy/8 F are prescribed to the GTV, with
67% coverage of the PTV. Clinicians- and patient-reported toxicity, along with efficacy
measures are registered in a Redcap database. Radiotherapy data are imported to
the “Danish national radiotherapy data bank (DcmCollab)” for analysis.
Results
The study started the inclusion of patients in October 2019. By November 2020, the primary goal was
reached (61 patients recruited). The protocol was amended due to excess
drop-out within the first year of follow-up and the recruitment population was
increased to a total of 120 patients. All patients are expected to be included
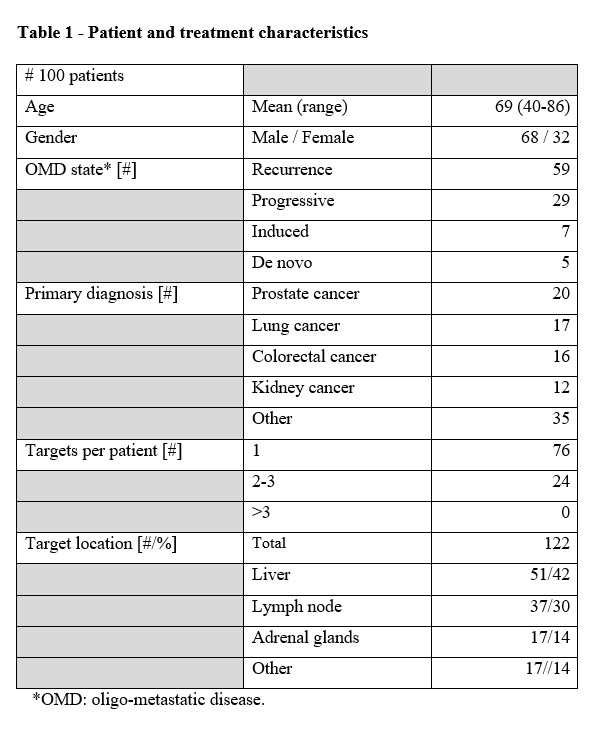
by the end of December 2021. In the first 100 patients, a total of 122 targets
have been treated with 111 treatment plans. Prostate -, lung- and colorectal
cancer are the most dominant primary diagnoses among the population (Table 1).
Most targets have been in the liver (42%), but lymph nodes (30%) and adrenal
glands (14%) also constitute a considerable part of the treated targets (Table
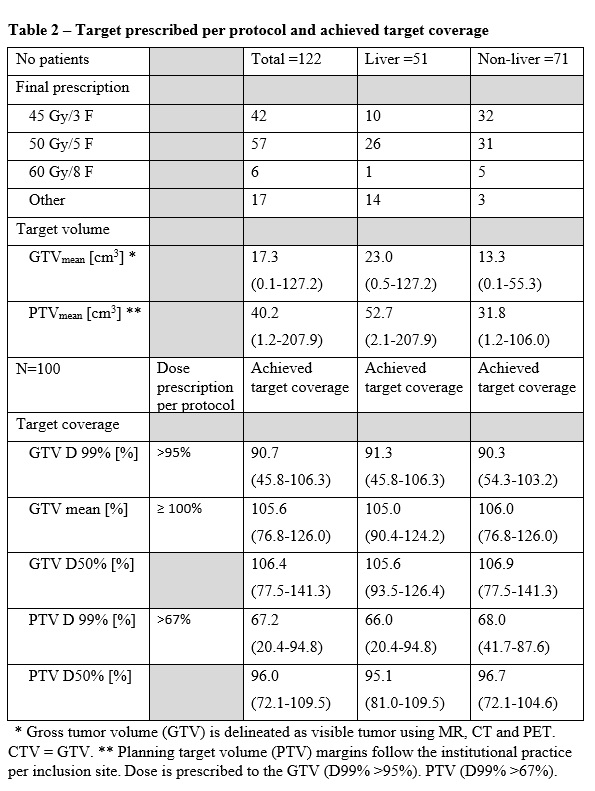
1). Most targets (86%) have been treated with the prescription dose per
protocol, whereas in 14% of the targets another prescription was chosen (Table
2). Targets in the liver were larger than non-liver (mean GTV volume = 23.0 cm3
vs. 13.3 cm3, P< 0.01 Mann-Whitney Test). Achieved target
coverage did not seem to be influenced by target location (liver vs. non-liver).
Conclusion
The SOFT trial is
successfully recruiting patients. The preliminary dosimetric results on the first
100 patients suggest that ablative doses can be achieved for high-risk targets both
inside and outside the liver. With continued effort to collect high-quality protocol
data, we hope to gain more experience in the referral pattern and clinical
outcome of patients with OMD. The SOFT protocol adheres to well-defined dose
prescriptions, and here the first step toward collecting clear and consistent
data on delivered target dose has been initiated.