Short-term toxicity outcomes after MR-guided SBRT for (peri-)pancreatic tumors on a 1.5T MR-linac
Hidde Eijkelenkamp,
The Netherlands
MO-0221
Abstract
Short-term toxicity outcomes after MR-guided SBRT for (peri-)pancreatic tumors on a 1.5T MR-linac
Authors: Hidde Eijkelenkamp1, Guus Grimbergen1, Hanne Heerkens1, Gert Meijer1, Lois Daamen1, Quintus Molenaar2, Hjalmar van Santvoort2, Beth Erickson3, William Hall3, Martijn Intven1
1University Medical Center Utrecht, Department of Radiotherapy, Utrecht, The Netherlands; 2Regional Academic Cancer Center Utrecht, Department of Surgery, Utrecht, The Netherlands; 3Medical College of Wisconsin, Department of Radiotherapy, Milwaukee, USA
Show Affiliations
Hide Affiliations
Purpose or Objective
Radiotherapy treatment of pancreatic cancer can
be challenging due to the close relationship between the tumor and radiosensitive
intestines and poor visibility of the upper abdomen with conventional treatment
methods. In recent years, with the introduction of MR-guided radiotherapy
(MRgRT), it became possible to deliver ablative doses to (peri-)pancreatic
tumors. In this multicenter cohort study, we report short-term toxicity from
our first cohort of patients with (peri-)pancreatic tumors with MR-guided
SBRT on a 1.5 T MR-linac.
Material and Methods
All patients with (peri-)pancreatic tumors treated
on the 1.5 T Unity MR-linac (Elekta AB, Stockholm, SE) between April 2019 and July 2021 at
University Medical Center Utrecht and Medical College of Wisconsin were
prospectively included in the Multi-OutcoMe EvaluatioN of radiation Therapy
Using the MR-Linac Study (MOMENTUM) (NCT04075305).
Toxicity was reported using clinician-reported outcome measurements defined by
the Common Terminology Criteria for Adverse Events (CTCAE) version 5. Toxicity
was assessed at baseline, and at 3, 6 and 12 months after treatment.
Results
Baseline data
was available for 61 patients with an average follow-up time of 5 months. The
median age was 70 years (range 38-91 years) and 35 patients were male (57%). Baseline
characteristics are summarized in Table 1. The most frequently delivered SBRT scheme
was 40 Gy in 5 fractions of 8 Gy. Most frequently reported toxicities after
treatment were fatigue, nausea, anorexia, and diarrhea. The course of these
toxicities over time is illustrated in Figure 1. Other grade 3 toxicity at 3
months follow up occurred in 2% of patients for vomiting; 2% for gastroparesis;
4% for malabsorption; 2% for weight loss; 2% for portal hypertension and 2% for
gallbladder obstruction. At six months, other grade 3 toxicity occurred in 3%
of patients for pancreatitis, and 7% for weight loss. At 12 months 8% had grade
3 weight loss. No treatment-related toxicities higher than grade 3 were
observed in our cohort.
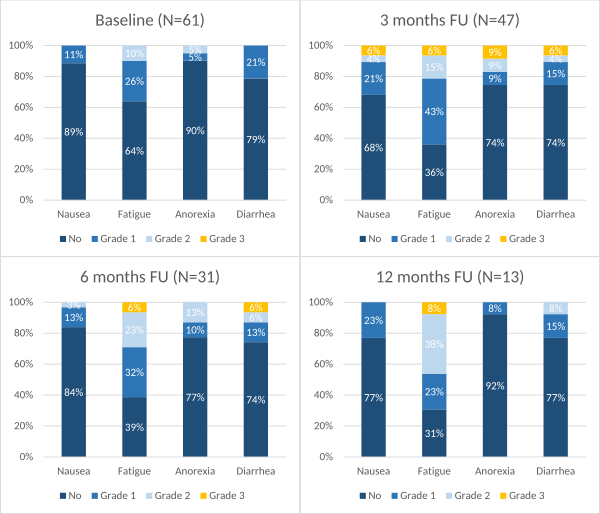
Figure 1. Toxicity outcomes
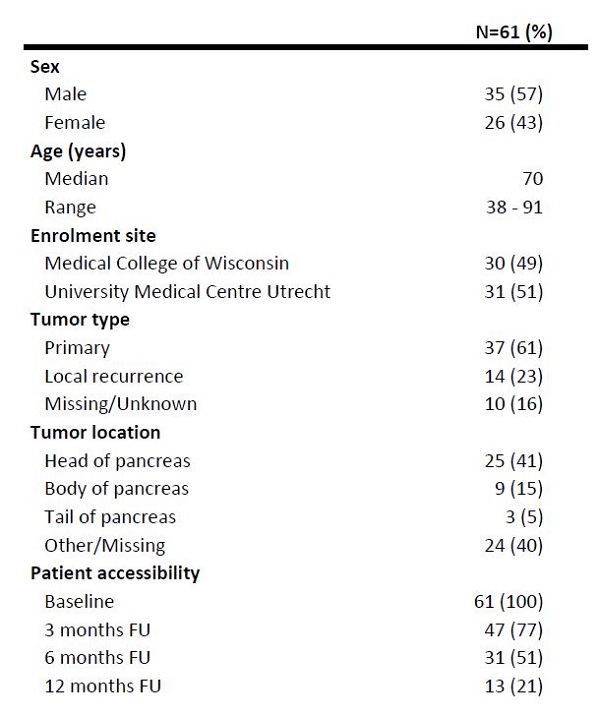
Table 1. Baseline characteristics
Conclusion
In this international
prospective cohort of patients with (peri-)pancreatic tumors, we observed a low
incidence of short-term major toxicity after MRgRT on a 1.5T MR-linac. The
results from this analysis will be updated before the ESTRO conference in 2022.