RT with hyperthermia in recurrent and radiation-induced sarcomas: preliminary results of NCT04398095
MO-0146
Abstract
RT with hyperthermia in recurrent and radiation-induced sarcomas: preliminary results of NCT04398095
Authors: Mateusz Spałek1, Aneta Borkowskia2, Michał Wągrodzki3, Patrycja Castaneda-Wysocka4, Piotr Rutkowski5
1Maria Sklodowska-Curie National Research Institute of Oncology, Department of Soft Tissue/Bone Sarcoma and Melanoma, Warsaw, Poland; 2Maria Sklodowska-Curie National Research Institute of Oncology, Department of Soft Tissue/Bone Sarcoma and Melanoma , Warsaw, Poland; 3Maria Sklodowska-Curie National Research Institute of Oncology, Department of Pathology and Laboratory Medicine, Warsaw, Poland; 4Maria Sklodowska-Curie National Research Institute of Oncology, Department of Radiology, Warsaw, Poland; 5 Maria Sklodowska-Curie National Research Institute of Oncology, Department of Soft Tissue/Bone Sarcoma and Melanoma , Warsaw, Poland
Show Affiliations
Hide Affiliations
Purpose or Objective
The role of perioperative treatment in radiation-induced and in-field recurrent sarcomas (RIS/IFRS) is unknown. Reirradiation may be associated with a risk of significant toxicity; thus, it is rarely used. We hypothesized that the combination of preoperative or definitive 12x 3 Gy radiotherapy (RT) with or without integrated 3.5 Gy to 42 Gy boost combined with regional hyperthermia twice a week will enable satisfactory local control without significant late toxicity in patients with RIS/IFRS.
Material and Methods
A prospective phase II, single-arm clinical trial was conducted. We included patients with locally advanced RIS/IFRS without distant metastases. Treatment combined three weeks of radiotherapy, four fractions per week, 3 or 3.5 Gy per fraction, with regional hyperthermia, followed by surgery or observation. The choice of the boost or no-boost regimen was based on resectability (Figure 1). The intervention would be deemed tolerable if significant RT-related (grade 3+ CTCAE 5.0) late adverse events occur in less than 20% of patients. We planned to enroll 20 patients based on Wilson’s method for calculation of confidence intervals.
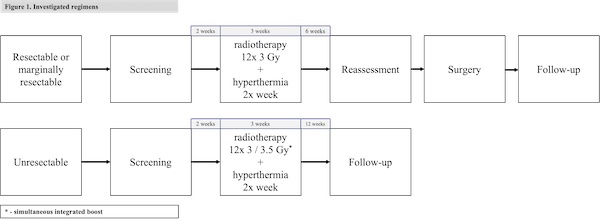
Results
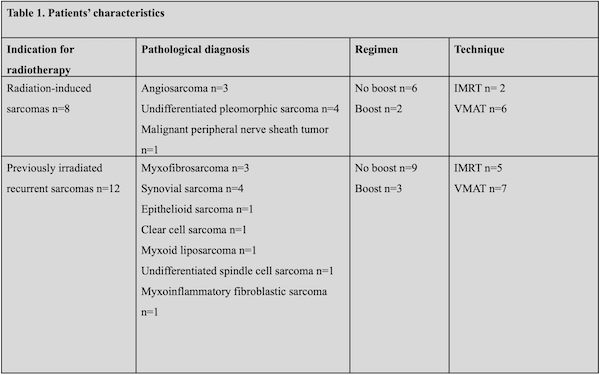
We recruited 20 patients. All patients completed the treatment without interruptions. Eight of them had RIS whereas twenty were diagnosed with IFRS. Patients’ characteristics were provided in Table 1. Twelve patients from planned 15 underwent surgery. Two patients with potentially resectable tumors did not undergo surgery due to COVID-related reasons. One patient preferred not to undergo surgery after the preoperative no-boost regimen.
The remaining five patients were deemed unresectable at the enrollment and received the simultaneous boost. In five patients who underwent resection, we observed extensive pathological response according to the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group recommendations for pathological examination and reporting, namely grade A in two cases and grade C in three cases. In four patients we observed complete radiological response.
The median follow-up was 13 months. In 14 patients we noted mild or moderate radiation dermatitis. One patient experienced grade 2 gastrointestinal toxicities. From the late toxicities, we observed restricted limb mobility (grade 1) in one patient and chronic skin ulceration (grade 2) in one patient. None of the patients who developed grade 3 or higher late toxicity.
Two patients who received the no-boost regimen and did not undergo resection developed local progression. One patient experienced borderline local relapse after surgery. None of the patients who received the boost regimen developed local progression. Three patients developed distant metastases. One patient was lost to follow-up.
Conclusion
Preliminary data suggest that the tolerance of the regimen is acceptable; however, data regarding late toxicity may change during the follow-up period. Boost may play a significant role in achieving local control in non-resected tumors.