Head and Neck Squamous Cell Carcinoma derived CAFs alter their phenotype after ionizing radation
Marleen Ansems,
The Netherlands
OC-0594
Abstract
Head and Neck Squamous Cell Carcinoma derived CAFs alter their phenotype after ionizing radation
Authors: Marleen Ansems1, Vera Mekers1, Anne Beerkens2, Minke Dotinga1, Konstantina Strepi2, Jimmie Honings3, Willem Weijs4, Gosse J. Adema1, Johannes HAM Kaanders1, Jan Bussink2
1Radboudumc, Radiation Oncology, Nijmegen, The Netherlands; 2Radboudumc, Radiation Oncology, Nijmegen , The Netherlands; 3Radboudumc, Otorhinolaryngology-Head and Neck Surgery, Nijmegen, The Netherlands; 4Radboudumc, Oral and Maxillofacial Surgery, Nijmegen, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Despite advances in treatment strategies of Head and Neck Squamous Cell Carcinoma (HNSCC), the chance of cure for recurrent HNSCC is poor. To increase the success rate of treatment strategies more knowledge regarding the interplay between cancer cells and other cell types in the tumour is essential. In particular cancer associated fibroblasts (CAFs) require special attention, since they make up the majority of cells in the tumour microenvironment of HNSCC. Currently, the majority of HNSCC patients will be treated with radiotherapy (RT). Despite the abundance of CAFs, the effect of RT on their function in HNSCC is largely unknown.
Material and Methods
The significance of CAFs in HNSCC was analysed using the TCGA database. The expression of CAF associated markers in HNSCC samples was compared to the expression in normal tissue samples and in HPV+ vs HPV- patients. The impact of ionizing radiation on murine and human HNSCC-derived CAFs was determined by RNA, flow cytometry and microscopic analyses.
Results
TCGA analysis revealed higher expression of CAF associated markers in HNSCC samples compared to normal tissue samples (Fig.1A) and higher expression in HPV- compared to HPV+ patients (Fig.1B). After RT, HNSCC-derived CAFs increase their cell size (Fig.1C,D), modulate expression of CAF-associated (Fig1E,F) and immune modulatory markers (Fig.1G). Our first results on patient-derived CAFs confirm that also human HNSCC derived CAFs are affected by RT (Fig.1H,I).
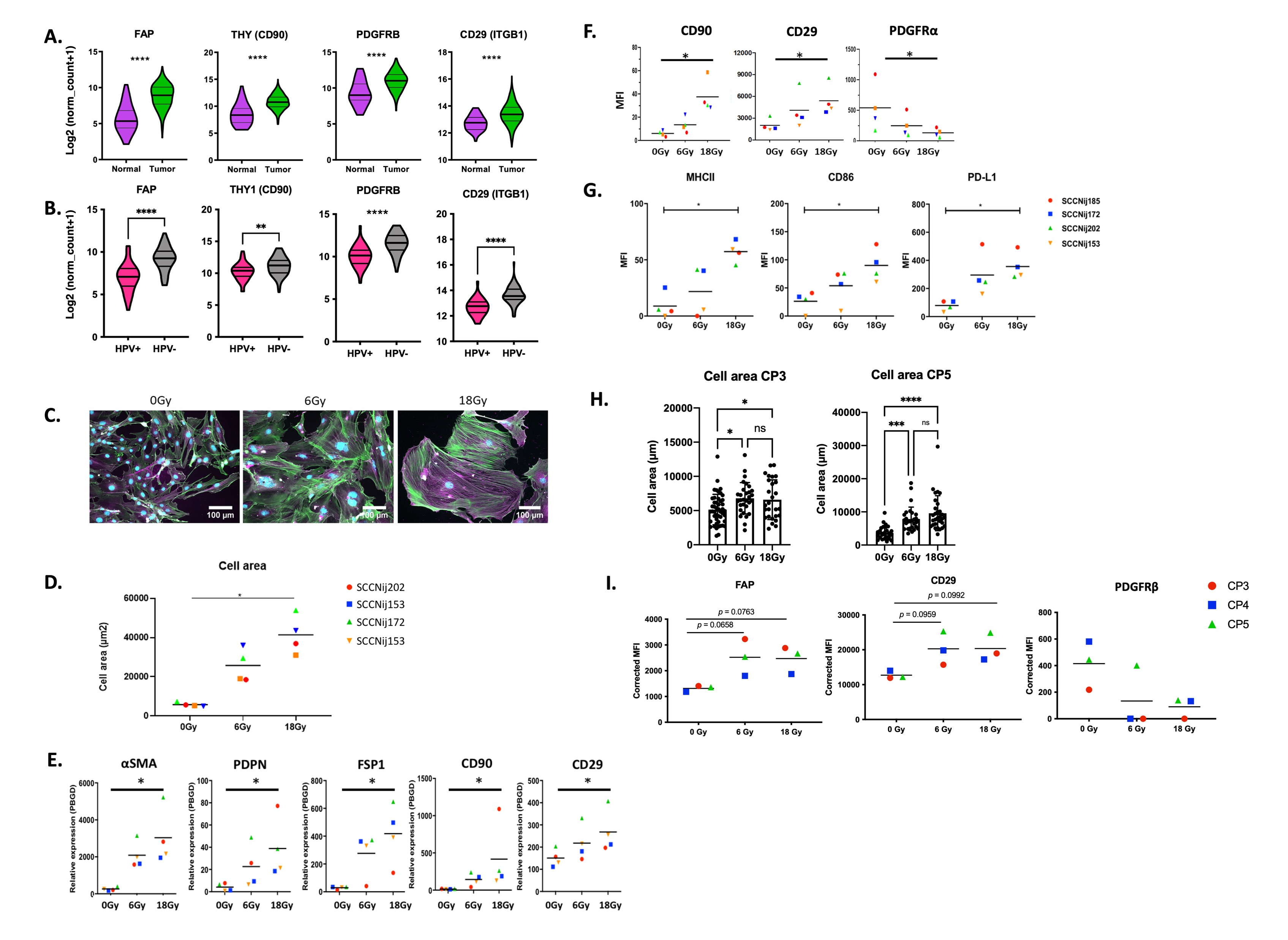
Fig.1 Radiotherapy affects HNSCC derived CAFs A) Expression in HNSCC tissues vs normal tissues B) Expression in HPV- and HPV+ HNSCC. CAFs from HNSCC patient-derived xenografts were irradiated and 3 days later stained for microscopy (αSMA (magenta), DAPI (blue) and total actin (green)). C. Cell surface area was determined with ImageJ D) The dots represent the mean cell area per HNSCC-derived CAF E) qPCR analysis, 3 days after irradiation F) FACS analysis 3 days after irradiation G) FACS analysis, 3 days after irradiation. H) CAFs isolated from HNSCC patients were subjected to radiation, 3 days later their cell area was determined with microscopy followed by ImageJ analysis. Each dot represents a cell, with mean and SD indicated. I) FACS analysis, 3 days after irradiation.
Conclusion
Our in silico analyses confirm the significant presence of CAFs in HNSCC, suggesting that CAFs contribute to the malignant phenotype of HNSCC. Our data show that RT modulates the phenotype of murine and human HNSCC-derived CAFs. It affects their morphology and the expression of markers that are important in steering anti-tumor responses. Current research focuses on the effect of RT on the function of CAFs in vivo and their subsequent effect to modulate the anti-tumour immune response and efficacy of immunotherapy.