SPECT Imaging of CAIX and monitoring of hypoxia after OXPHOS inhibition in murine tumor models
Daan Boreel,
The Netherlands
OC-0593
Abstract
SPECT Imaging of CAIX and monitoring of hypoxia after OXPHOS inhibition in murine tumor models
Authors: Daan Boreel1, Paul Span2, Hans Peters2, Annemarie Kip3, Milou Boswinkel3, Gosse Adema2, Sandra Heskamp3, Johan Bussink2
1Radboud University Medical Center, Radiation oncology, Nijmegen, The Netherlands; 2Radboud University Medical Center, Radiation Oncology, Nijmegen, The Netherlands; 3Radboud University Medical Center, Medical Imaging, Nijmegen, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Limited diffusion of
oxygen into remote tumor areas and oxygen consumption through oxidative
phosphorylation (OXPHOS) leads to hypoxia in most solid tumors. This scarcity
of oxygen is known to induce radioresistance, but can also have a disrupting
effect on the anti-tumor immune response. Therefore,
we investigated the potential of
OXPHOS inhibition to relieve tumor hypoxia by decreasing the oxygen
consumption. Furthermore, we
developed a radiolabeled antibody which recognizes murine CAIX ([111In]In-DTPA-mCAIX),
an enzyme upregulated by cancer cells under chronic hypoxic conditions, to monitor chronic hypoxia in syngeneic mouse models.
Material and Methods
Several syngeneic murine cell lines and tumor models on a C57Bl/6
background were used (B16ova, MOC1,
MC38 and GL261). In vitro oxygen
consumption of these tumor cells was measured using the Agilent XF Seahorse Analyzer
before and after treatment with the OXPHOS inhibitor IACS-010759. The in vivo tumor microenvironment of B16ova
and MOC1 tumors, treated (10mg/kg IACS-010759) and vehicle-treated (0.5%
methylcellulose), was characterized by immunohistochemistry. The
biodistribution of [111In]In-DTPA-mCAIX
was measured by ex vivo radioactivity
counting and in vivo SPECT imaging
comparing different antibody doses and time points post injection. Intratumoral
distribution of tracer uptake was visualized using autoradiography. Image
analysis was performed by parametric mapping and zonal analysis in ImageJ.
Results
The data show that
mitochondrial complex I inhibitor IACS-010759 inhibited oxygen consumption in a
dose dependent manner in several tumor cell lines in vitro. Furthermore, diffusion limited hypoxia in vivo is reduced up to 200μm from perfused blood vessels by IACS-01079
treatment (10mg/kg) in MOC1 and B16ova tumors (fig 1). This can be
visualized by staining pimonidazole as well as CAIX. In vitro, [In111]-DTPA-mCAIX showed specific binding to B16ova cells when
cultured at 1% O2 (9.3±1.2%), but not to cells cultured at 20% O2
(0.8±0.04%). In vivo, CAIX expression
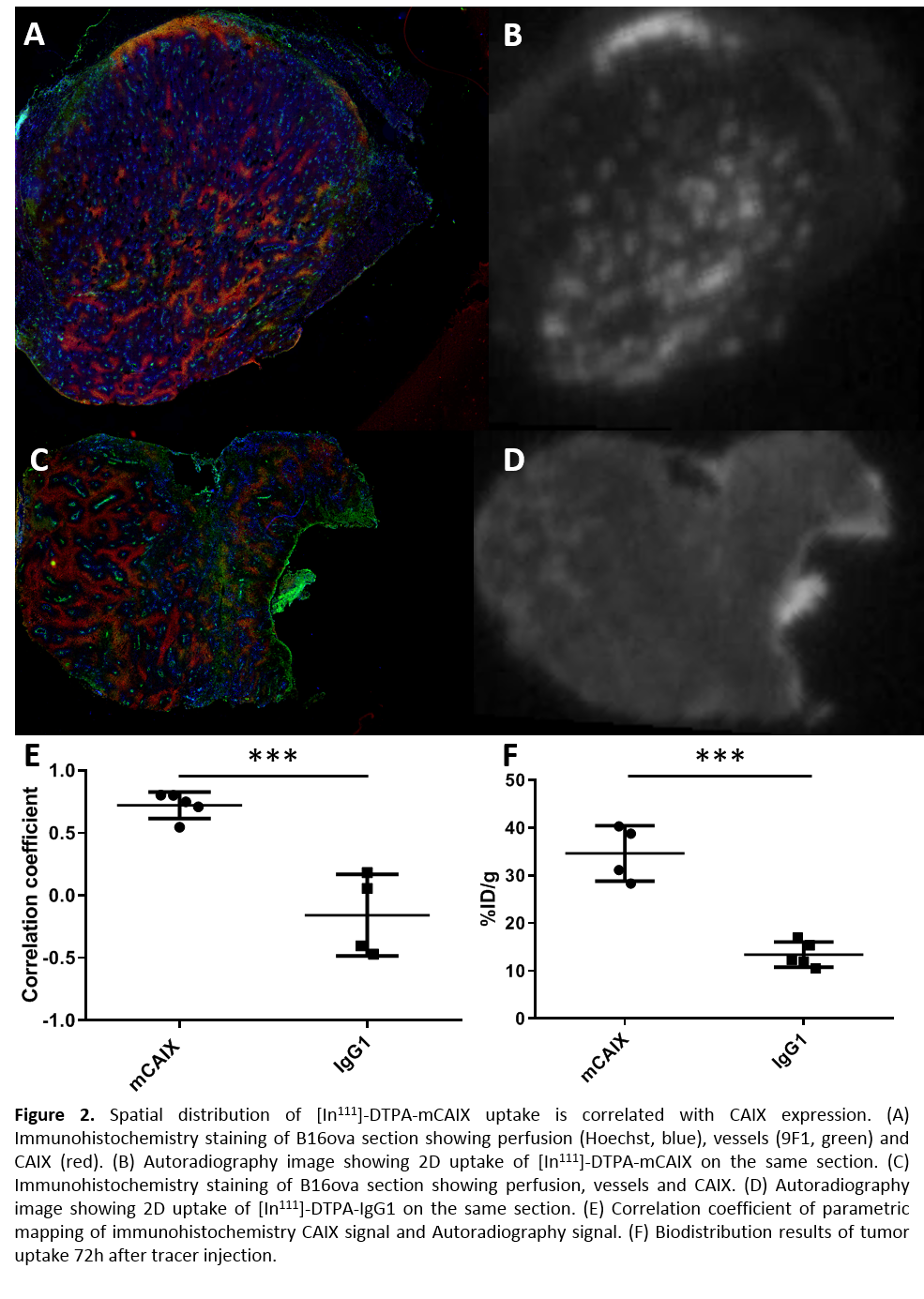
could be visualized by SPECT using [In111]-DTPA-mCAIX. Radiotracer uptake in the tumor was significantly
higher compared with uptake of isotype control tracer [In111]-DTPA-IgG1
(34.6±5.8 vs. 13.4±2.7 %ID/g) (fig 2F). Autoradiography and immunohistochemistry
of tumor sections showed a strong spatial correlation of CAIX with [In111]-DTPA-mCAIX
(r=0.72±0.11) and not with [In111]-DTPA-IgG1
(-0.16±0.33)(fig 2A-E). 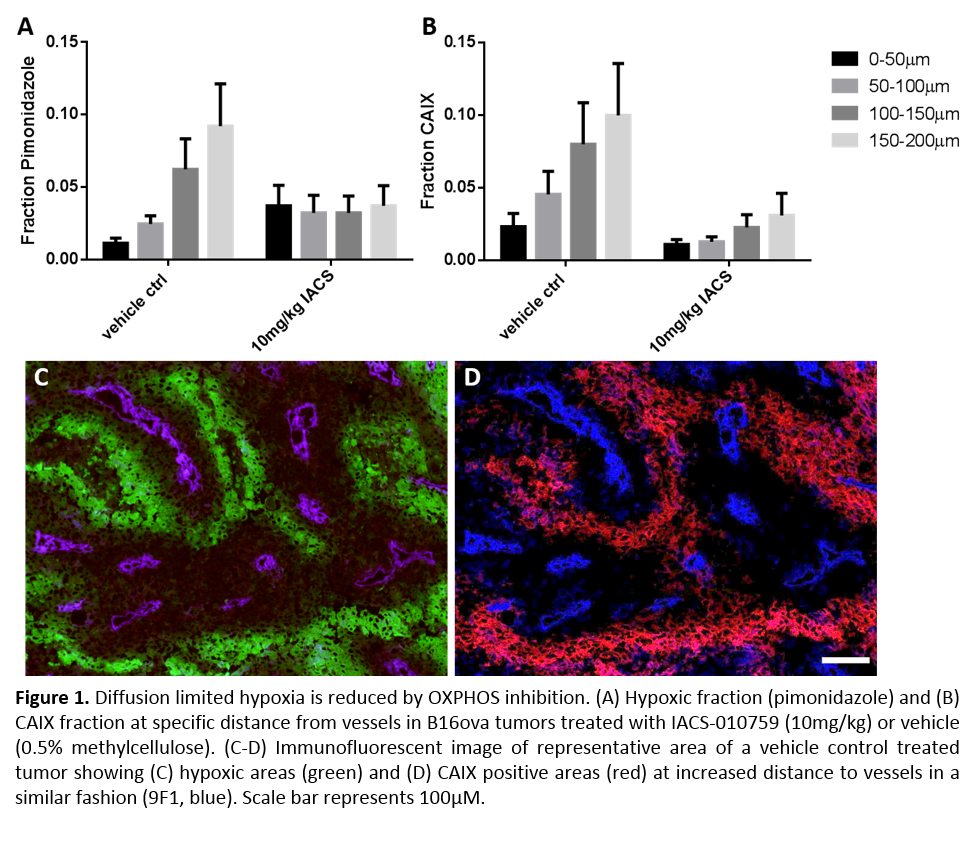
Conclusion
OXPHOS inhibition
decreases oxygen consumption in several tumor cell lines in vitro and
decreases diffusion limited hypoxia in
vivo. Furthermore, the hypoxia related marker CAIX can be used to visualize
hypoxic areas in syngeneic mouse models using the SPECT-radiotracer [In111]-DTPA-mCAIX.
In the future, this technique could be used to distinguish hypoxic from
non-hypoxic tumors before or during OXPHOS inhibition treatment and thereby help
optimizing this strategy to relieve tumor hypoxia and improve immuno- and
radiotherapy efficacy in preclinical models.