High sensitivity of PROMs detecting boost-vs-no-boost during breast radiotherapy
OC-0587
Abstract
High sensitivity of PROMs detecting boost-vs-no-boost during breast radiotherapy
Authors: Gerd Heilemann1, Andreas Renner1, Daniela Kauer-Dorner1, Inga-Malin Simek1, Stefan Konrad1, Dietmar Georg1, Joachim Widder1
1Medical University of Vienna, Department for Radiation Oncology/Comprehensive Cancer Center, Vienna, Austria
Show Affiliations
Hide Affiliations
Purpose or Objective
To
evaluate an in-house developed patient-reported outcome measures (PROM)
platform to assess on-treatment radiation related toxicities of breast cancer
patients.
Material and Methods
Starting
in November 2020, every eligible patient with breast cancer was enrolled on a
voluntary basis into the IRB-approved Patient Experience Data in Radiation
Oncology (PEDRO) study (EK 2184/2019). Patients received hypofractionated
whole-breast irradiation to 40 Gy in 15 fractions, followed by a risk-adapted sequential
boost of 10 Gy in four fractions if indicated. Consenting participants answered
a predefined set of questions from the PRO-CTCAE catalogue. Surveys were
carried out weekly on tablets in the out-patient clinic for the duration of the
treatment. The PROM application, the app-server and database infrastructure
were all in-house developed. The PROM data were directly linked to relevant treatment
data from the oncology information system. Differences between the two cohorts
(w/o boost) were analyzed for significant differences with respect to the
endpoints: itching, radiation skin reaction, skin darkening, and breast
swelling and tenderness.
Results
A
total of 330 patients with breast cancer participated in this real-world PROM
setting (40% of all patients with breast cancer treated in this period),
resulting in 1527 individual completed questionnaires. 177 patients (54%) were
treated with a sequential boost, 153 patients (46%) received no boost. The
maximum reported side effects at the respective last fractions were
significantly higher in the boosted group for different clinical endpoints
(Fig. 1): itching (p<0.001), radiation skin reaction (p<0.001), skin
darkening (p<0.001), and breast swelling and tenderness (p=0.05). These
differences generally disappeared at the sensitivity analysis, when the surveys
at 40 Gy were compared for both groups. Baseline PROMs were also equal among
groups (p=0.40, p=0.42, p=0.88, p=0.73, respectively). An analysis of the
significance in differences with growing sample size (Fig. 2) suggested that dichotomy
stabilized at around 100 patients for radiation skin reaction and skin
darkening, as well as 200 patients for itching. Data suggests that the
reporting of breast swelling and tenderness requires larger sample sizes (>330).
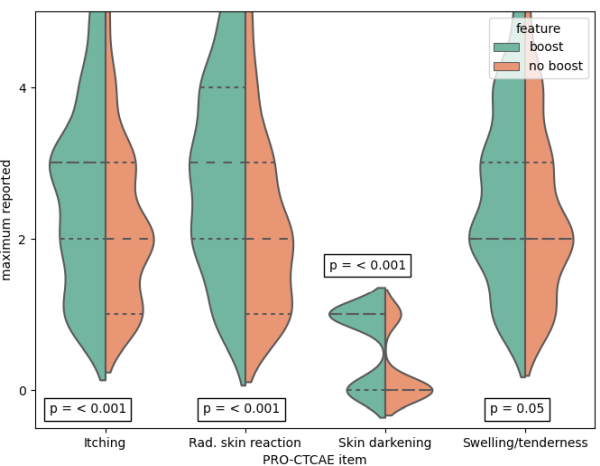
Fig1: Comparison patients that received
boost (green) with patients without boost (red) with respect to the maximum
reported value for the endpoints (dashed = quatiles).
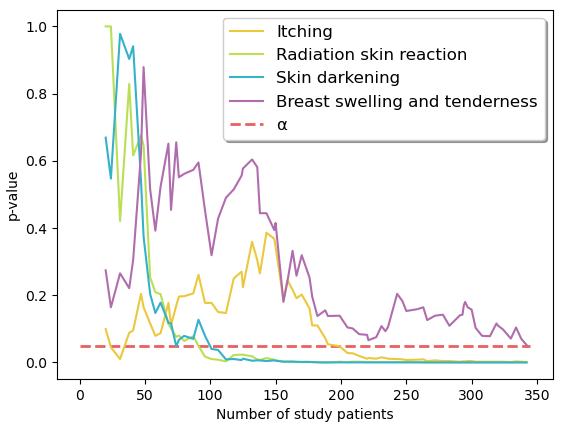
Fig2: Change of p-value (boost vs. no boost) over
increasing number of sample size.
Conclusion
PROMs
were successfully implemented into clinical routine and yielded a surprisingly high
sensitivity detecting discrete dose-dependent radiation reactions in the breast-irradiation
PEDRO pilot module. The extension of this in-house developed tool is prone to
provide valuable information on subtle radiation induced toxicities and will help
guide knowledge-based improvements of treatment planning and monitor changes in
treatment schedules such as e.g., extreme hypofractionation.