Deep Learning-based segmentation of prostatic urethra on CT scans for treatment planning
Laura García-Elcano,
Spain
OC-0770
Abstract
Deep Learning-based segmentation of prostatic urethra on CT scans for treatment planning
Authors: Laura García-Elcano1,2, Eugenia Mylona3,4, Oscar Acosta3, Thibaut Lizée3, Khemara Gnep3, Renaud de Crevoisier3, Javier Pascau5
1Universidad Carlos III de Madrid, Departamento de Bioingeniería e Ingeniería Aeroespacial, Madrid, Spain; 2Centro de Investigación Médica Aplicada, Clínica Universidad de Navarra, Madrid, Spain; 3Université de Rennes 1, CLCC Eugène Marquis, INSERM, LTSI - UMR 1099, Rennes, France; 4University of Ioannina, Department of Materials Science and Engineering, Unit of Medical Technology and Intelligent Information Systems, Ioannina, Greece; 5Universidad Carlos III de Madrid, Departamento de Bioingeniería e Ingeniería Aeroespacial, Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain
Show Affiliations
Hide Affiliations
Purpose or Objective
Urethra damage has been shown to be highly
associated with urinary toxicity after prostate cancer radiotherapy.
Identifying the urethra is therefore essential for efficient treatment
planning, but is still challenging as this structure is completely invisible in
the CT scans. Although atlas-based approaches have been proposed, their long
execution time limits their clinical application. Here, we propose a
Deep Learning (DL) based method for segmenting the intra-prostatic urethra from CT and compare it with a state-of-the-art model in terms of quality and time. The
method exploits nearby OARs contours to anatomically guide the model, which
learns the location of the urethra directly from computed meaningful Distance
Maps (DMs).
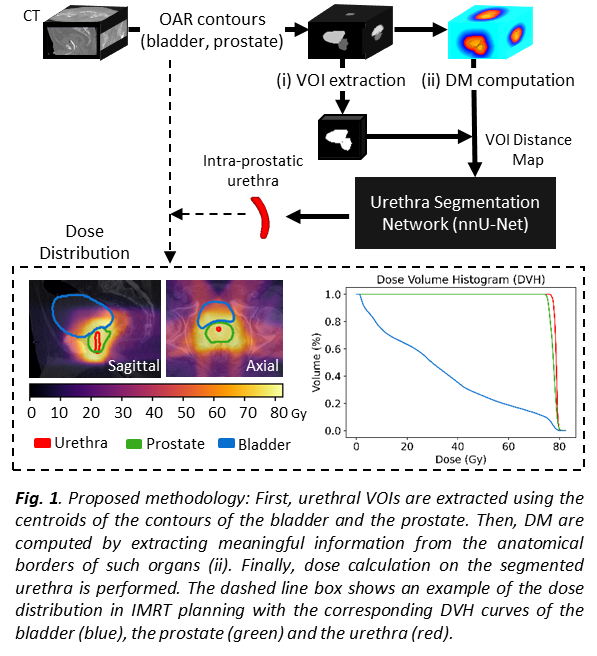
Material and Methods
Manual contours of the prostate, bladder and
urinary catheters from 55 CT images of patients treated with brachytherapy and
rigidly registered to a common space were randomly divided into training (80%)
and testing (20%) datasets. The binary masks of the prostate and the bladder
were employed to extract fixed volumes of interest (VOIs) and calculate DMs.
For that, different combinations of the Euclidean distance transform and the
Laplacian descriptor to the centroid of each organ were considered. These
distance images were then used to train the so-called nnU-Net, a DL-based
segmentation algorithm that includes automatic configuration and 5-fold
cross-validation. To evaluate the concordance between the segmented urethra and
the ground-truth catheter we measured Dice similarity coefficient (DSC),
average surface distance (ASD), centerline distances (CLD) and the percentage
of centerline lying in a certain radius of the catheter (PWR). The segmentation
framework (Fig. 1) was applied to an independent database of 32 patients
treated with IMRT at 78/80 Gy to quantify dose on the urethra.
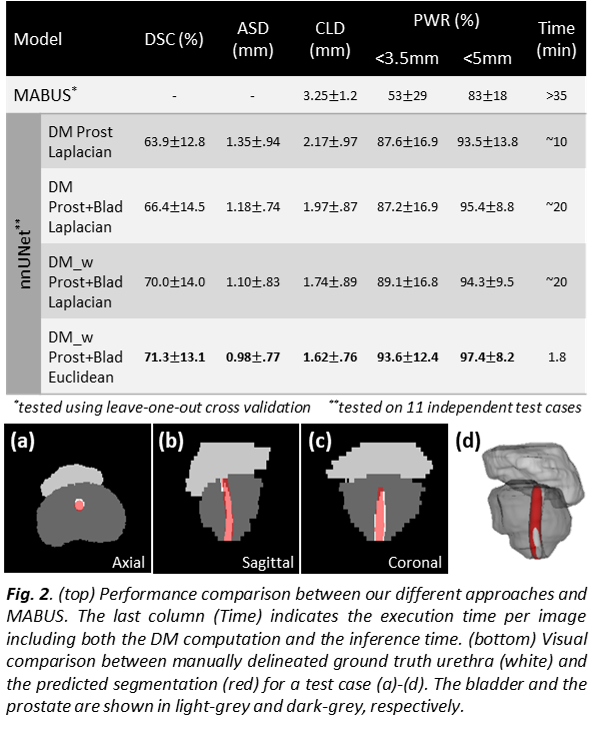
Results
The test performance of our approaches is
summarized in Fig. 2. For all the metrics, the nnU-Net shows lower mean error
values and lower variability compared to an existing multi-atlas based strategy
(MABUS), with a significant improvement of both the CLD (1.63mm) and PWR within
a radius <3.5mm (41%) and <5mm (14%) for our best model using the
Euclidean distance and a weighted approach. Each 3D CT image, including DM
computation and inference time, took an average runtime of 1.8 mins on a
Nvidia RTX 8000 GPU. In IMRT, the urethra received higher doses (mean D95%urethra
-dose to 95% of the urethra- was 77.6Gy) than the whole prostate (mean D95%prostate=76.6Gy).
Conclusion
We propose a DL-based solution that provides
accurate and fast segmentation of the urethra. Our results showed that DL takes
advantage of DMs to predict invisible regions in the CT scans, such as the urethra.
The inputs of the proposed framework are only segmentation masks, so the
translation into the MR domain would be straightforward. This method can be
potentially applied to the clinical workflow to devise personalized treatment
with reduced urinary toxicity in prostate radiation therapy.