Development and validation of a population-based colorectal model for radiation therapy dosimetry
OC-0939
Abstract
Development and validation of a population-based colorectal model for radiation therapy dosimetry
Authors: Constance Owens1,2, Bastien Rigaud3, Ethan Ludmir4,5, Aashish Gupta3,2, Suman Shrestha2,1, Arnold de la Cruz Paulino4, Christine Peterson5,2, Stephen Kry1,2, Susan Smith1, Kristy Brock3,1, Tara Henderson6, Rebecca Howell1,2
1The University of Texas MD Anderson Cancer Center, Department of Radiation Physics, Houston, USA; 2MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences, Graduate Program in Medical Physics, Houston, USA; 3The University of Texas MD Anderson Cancer Center, Department of Imaging Physics, Houston, USA; 4The University of Texas MD Anderson Cancer Center, Department of Radiation Oncology, Houston, USA; 5The University of Texas MD Anderson Cancer Center, Department of Biostatistics, Houston, USA; 6The University of Chicago, Department of Pediatrics, Chicago, USA
Show Affiliations
Hide Affiliations
Purpose or Objective
There are no dose-response models
establishing relationships between colorectal doses or dose-volume metrics and late
colorectal sequelae (such as subsequent malignant neoplasms) in childhood
cancer survivors. Such models do not exist because these
studies require large cohorts with decades of follow-up. Consequently, these
cohorts are largely comprised of patients treated
in the pre-CT era of radiation therapy (RT) where organ dose calculations were
not possible. Thus, it is common practice in late effects studies to reconstruct
survivors’ RT on computational phantoms to estimate organ doses. However, the
Late Effects Group computational phantom, which has been used for hundreds of late effects
studies over several decades, does not have a colorectal model. Here, we aimed
to (1) add a colorectal model that incorporates pediatric anatomical variations
and (2) validate the geometric and dosimetric accuracy of the model across the
typical age range of pediatric RT patients.
Material and Methods
Whole-body non-contrast CT scans
of 114 pediatric patients (age range: 2.1-21.6 years, 74 males, 40 females)
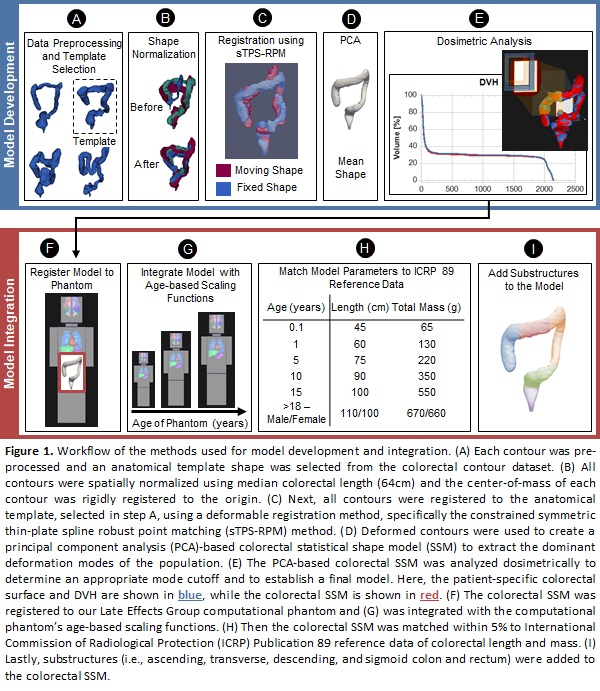
were retrospectively selected. Manual colorectal contours were reviewed and approved by two
radiation oncologists. 1 patient was used for the anatomical template, 103 for
training and 10 for testing. All contours were normalized using median
colorectal length and registered to an anatomical template using the
constrained symmetric thin-plate spline robust point matching method. Deformed
contours were used to create a principal component analysis-based colorectal statistical
shape model to extract the dominant deformations of the population. Geometric
accuracy was validated using the Dice similarity coefficient (DSC),
distance-to-agreement (DTA), and Hausdorff distance (HD) between the patient-specific
and model contours for 10 test patients. Dosimetric accuracy was validated
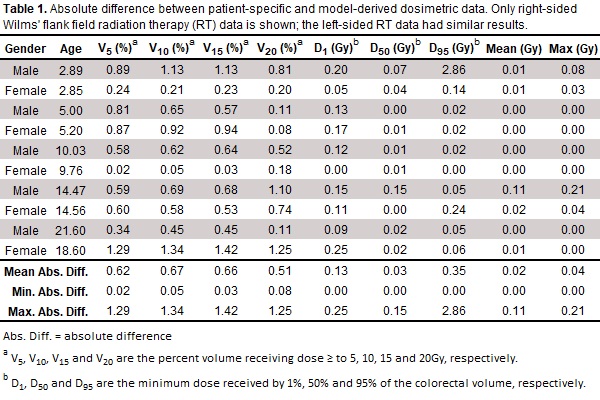
using standard Wilms’ tumor 3D conformal RT plans on the test patients. We
computed colorectal RT dose data (mean,
max, V5, V10, V15, V20, D1,
D50, and D95) and compared the patient-specific data
with that of the colorectal model (Table 1). Workflow illustrated in Fig. 1.
Results
Using the colorectal model on the unseen 10 test
patients, the mean (min-max) DSC, DTA and HD between the patient-specific and
reconstructed model contours was 0.89 (0.85-0.91), 2.1mm (1.7-3.0), and 8.6mm (5.7-14.3),
respectively. For the Wilms’ RT plans, the average absolute difference in mean
and max dose, was 0.02Gy (0.00-0.11) and 0.03Gy (0.00-0.21), respectively. For V5,
V10, V15, and V20 all absolute differences
were within 1.6%. For D1, D50, and D95 all
absolute differences were within 2.9Gy.
Conclusion
We demonstrated that our colorectal statistical shape model can reconstruct unseen shapes with good
accuracy and be used for accurate dose reconstruction. The model will be integrated (Fig. 1F-I) into the Late Effects Group computational phantom and be used to reconstruct colorectal doses
for studies of RT-related colorectal late effects.