Radiotherapy in patients receiving anthracyclines: phase 3 SAFE trial (NCT2236806) interim analysis
OC-0935
Abstract
Radiotherapy in patients receiving anthracyclines: phase 3 SAFE trial (NCT2236806) interim analysis
Authors: Icro Meattini1, Carlotta Becherini1, Luca Visani1, Isacco Desideri1, Gabriele Simontacchi1, Vieri Scotti1, Beatrice Detti1, Giulio Francolini1, Mauro Loi1, Daniela Greto1, Pierluigi Bonomo1, Monica Mangoni1, Giuseppe Barletta2, Lorenzo Livi1
1University of Florence, Department of Experimental and Clinical Biomedical Sciences, Florence, Italy; 2Careggi University Hospital, CardioThoracic and Vascular Department, Florence, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
Several studies have evaluated cardioprotective strategies to prevent myocardial dysfunction in patients receiving cardiotoxic therapies. The SAFE trial (ClinicaTrials.gov identifier: NCT2236806) is a four-arm, randomized, phase 3, double-blind, placebo-controlled study. This is a subgroup analysis focused on the impact of postoperative breast radiation therapy (RT) of the pre-specified interim analysis on the first 174 patients who had completed cardiac assessment at 12-month.
Material and Methods
Patients were eligible for trial inclusion if they had indication to primary or postoperative systemic therapy using an anthracycline-based regimen. Cardioprotective therapy (bisoprolol, ramipril, or both drugs, as compared to placebo) was administered for 1 year from the initiation of chemotherapy or until the end of trastuzumab therapy. The primary endpoint was defined as detection of any subclinical impairment (worsening ≥10%) in myocardial function and deformation measured with standard and 3-dimensional (3D) echocardiography, left ventricular ejection fraction (LVEF) and global longitudinal strain (GLS).
Results
At 12-month, 3D-LVEF worsened by 4.4% in placebo arm and 3.0%, 1.9%, 1.3% in ramipril, bisoprolol, ramipril plus bisoprolol arms, respectively (P = .005). GLS worsened by 6.0% in placebo arm and 1.5%, .6% in ramipril, and bisoprolol arms, respectively; whereas it was unchanged (.1% improvement) in ramipril plus bisoprolol arm (P < .001). Concerning differences in 3D-LVEF changes from baseline to end of treatment, bisoprolol-containing arms showed significant benefit in patients not receiving RT (P = .09), in patients receiving right-sided breast RT (P = .0001), and with lesser extent, in patients receiving left-sided RT (P = .041). No significant benefit was shown in ramipril-containing arms. Concerning differences in GLS changes from baseline to end of treatment, bisoprolol-containing arms showed significant benefit in patients not receiving RT (P = .0001) and in patients receiving right-sided breast RT (P = .0001), while no benefit was shown in patients receiving left-sided breast RT (P = .270). Ramipril-containing arms showed significant benefit in patients not receiving RT (P = .035) and in patients receiving left-sided breast RT (P = 0.14), while no benefit was shown in right-sided breast RT (P = .260).
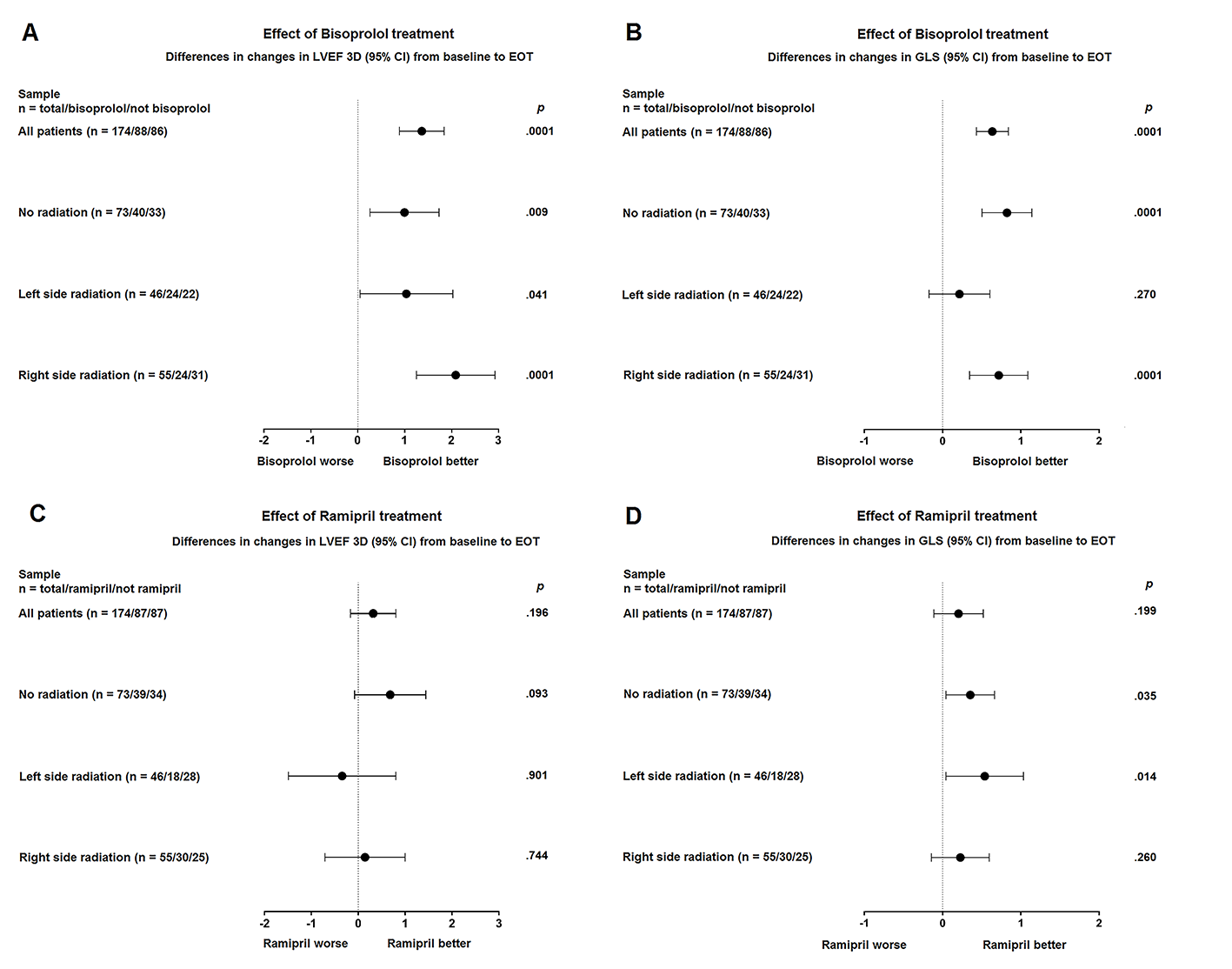
Conclusion
At the interim analysis, cardioprotective pharmacological strategies in patients affected by breast cancer receiving an anthracycline-based chemotherapy are well tolerated and seem to protect against cancer therapy-related LVEF decline and heart remodeling. This favorable effect seems to be reduced in patients receiving postoperative left-sided breast RT, thus calling for further investigations on potentially radiation-related early subclinical heart damage.