A comparison of doctor and therapeutic radiographer (RTT) prostate contours on T2 weighted MRI
Gillian Smith,
United Kingdom
OC-0465
Abstract
A comparison of doctor and therapeutic radiographer (RTT) prostate contours on T2 weighted MRI
Authors: Gillian Smith1, Alex Dunlop1,2, Sophie Alexander1, Helen Barnes1, Trina Herbert1, Rebekah Lawes1, Julia Murray1,3, Angela Pathmanathan1,3, Priyanka Patel1,3, Kobika Sritharan1,3, Nora Sundahl1,3,4, Rosalyne Westley1,3, Alison Tree1,3, Helen McNair1,3
1Royal Marsden NHS Foundation Trust, Radiotherapy, London, United Kingdom; 2Institute of Cancer Research, Physics, London, United Kingdom; 3Institute of Cancer Research, Radiotherapy, London, United Kingdom; 4Ghent University Hospital, Radiation Oncology, Ghent, Belgium
Show Affiliations
Hide Affiliations
Purpose or Objective
MR-linac (MRL) systems enable daily online re-contouring and
treatment plan re-optimisation. Currently this requires the presence of a doctor,
however, it is anticipated that therapeutic radiographers (RTTs) will take over
the re-contouring section of the workflow, alleviating the presence of the doctor.
To facilitate this, a RTT prostate and seminal vesicles (SVs) contouring training programme was developed.
We investigated whether RTT contours were within the inter-observer range of
the doctors’ contours.
Material and Methods
Five RTTs and five doctors contoured the prostate and SVs on
10 T2-weighted MRL-acquired MRIs,
from 10 patients, on the Monaco treatment planning system (Version: 5.59.02
Research, Elekta, Stockholm, Sweden). For each RTT and doctor, two clinical
target volumes (CTV) were created following the PRISM (NCT03658525) and PACE (NCT01584258) protocol:
CTVpsv: prostate plus 1cm SVs
CTVsv: prostate plus 2cm SVs
Simultaneous truth and performance level estimation (STAPLE)
structures were created using the five doctors’ contours to create “gold
standard” CTVpsv and CTVsv volumes. Each RTT and doctor structure was then
compared with the STAPLE structure using ADMIRE (Version: research 2.0 Elekta,
Stockholm, Sweden) to generate a dice similarity coefficient (DSC). The DSC
results were compared using a Mann Whitney U test. The inter-observer range of
volumes was extracted.
Results
Fifty CTVpsv and fifty CTVsv structures were created for
both doctors and RTTs. The median DSC for RTT and doctor structures
compared to the STAPLE were not significantly different (p=0.18) (Table
1).
|
|
Median (range) DSC
|
|
|
Doctors
|
RTTs
|
|
CTVpsv
|
0.92 (0.88 –
0.97)
|
0.92 (0.86 –
0.96)
|
|
CTVsv
|
0.92 (0.86 –
0.97)
|
0.91 (0.83 –
0.96)
|
Table 1. Dice
similarity coefficient for RTT and doctor contours compared to a STAPLE contour.
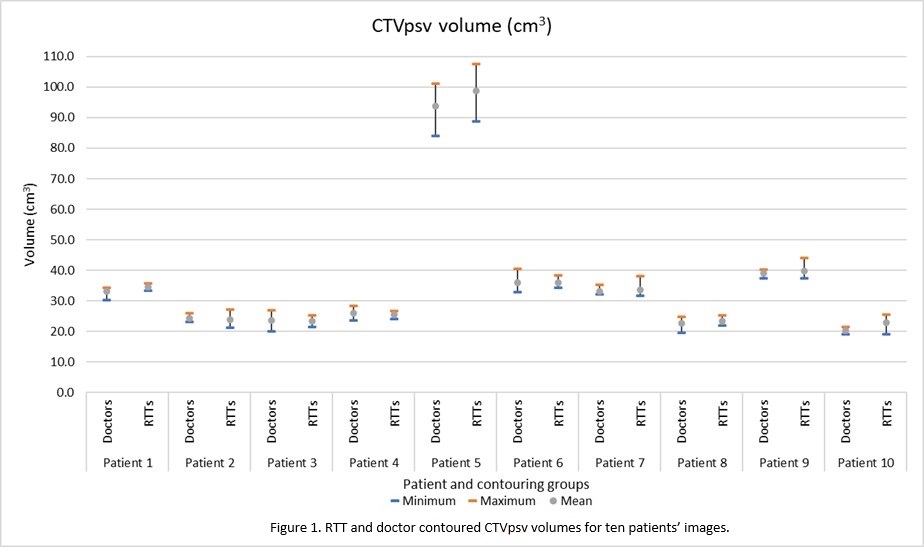
CTVpsv; Of the 50 CTVpsv RTT structures, 30 volumes
were within the doctors’ volume range (Figure 1). Six were smaller than the minimum doctor’s
volume and 14 were larger than the maximum doctor’s volume. Of the six smaller,
four were ≤1%
smaller (0.1cm3 to 0.3cm3) and the remaining two were 7% (1.5cm3)
and 8% (1.8cm3) smaller respectively.
CTVsv; Of the 50 CTVsv RTT structures, 35 were
within the doctors’ volume range. Three were smaller than the minimum doctor’s
volume and 12 were larger than the maximum doctor’s volume. Of the three, one
was < 1% smaller (0.3 cm3), one 3.4% smaller (0.9 cm3)
and one 7.5% smaller (1.9 cm3).
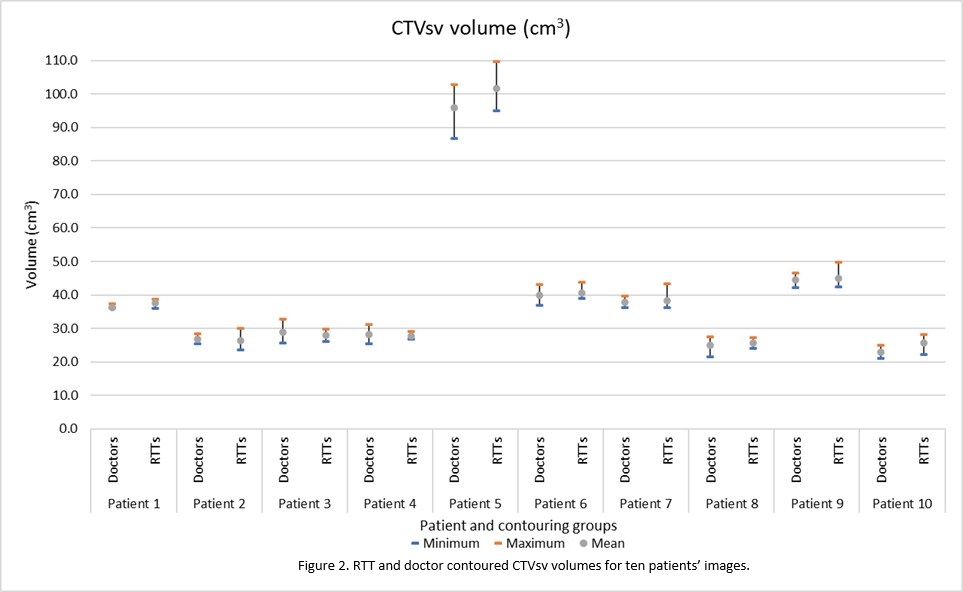
On visual inspection, the variation at the base and
apex contributed to the largest differencein contour volume.
Conclusion
The majority of RTTs’ contours are within the range of doctors’
inter-observer variability. The dosimetric and clinical significance of those
outside the range will be determined prior to RTTs replacing doctors in the
online adaptive workflow. Further investigations of RTT-led online contouring on the MRL are ongoing.