Proton-therapy and concurrent chemotherapy in stage III NSCLC: effects on hematological toxicity
Francesco Cortiula,
The Netherlands
OC-0438
Abstract
Proton-therapy and concurrent chemotherapy in stage III NSCLC: effects on hematological toxicity
Authors: Francesco Cortiula1, Lizza Hendriks2, Michelle Steens3, Safiye Dursun4, Gerben Bootsma3, Richard Canters1, Ilaria Rinaldi1, Vicki Trier Taasti5, Ruud Houben5, Kobe Reynders1, Stéphanie Peeters1, Antonio Angrisani1, Djoya Hattu1, Dirk De Ruysscher5
1Maastricht University Medical Center+, Department of Radiation Oncology (MAASTRO), Maastricht, The Netherlands; 2Maastricht University Medical Center+, Department of Pulmonary Diseases, Maastricht, The Netherlands; 3Zuyderland Medical Centre, Department of Pulmonary Diseases, Geleen, The Netherlands; 4Maastricht University Medical Center+, Maastricht, Department of Pulmonary Diseases, Maastricht, The Netherlands; 5Maastricht University Medical Center+, Department of Radiation Oncology (MAASTRO) , Maastricht, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
The primary aim of this
study was to assess whether intensity modulated proton therapy (IMPT), compared
to intensity modulated photon therapy (IMRT) can reduce hematological toxicity
in patients (pts) treated with concurrent chemotherapy (CCRT) for stage III Non-Small
Cell Lung Cancer (NSCLC).
Material and Methods
Retrospective
data completion and analysis of a 2-center prospectively collected series of patients
with stage III NSCLC. Pts with stage III NSCLC, receiving CCRT between 06.16-02.21,
and staged with FDG-PET-CT and MRI brain were eligible. Primary endpoint:
incidence of lymphopenia grade (G) ≥3 in IMPT vs IMRT treated pts. Secondary
endpoints: the effects of IMPT in terms of pts’ recovery after CCRT and other
toxicities incidence; to investigate the association between lymphopenia and the
bone marrow radiation volumes (RVs). Main exclusion criteria: invasive cancer
diagnosis in previous two years, previous thoracic RT and RT dose >66 Gy. Bone
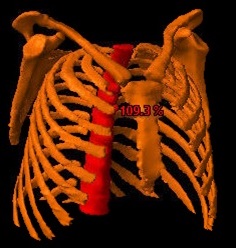
marrow was defined as the sum of the following structures: sternum, scapulae,
clavicles, thoracic vertebrae and ribs, delineated until the level of T12 (figure 1). RVs were retrieved from
regular care RT planning. Categorical variables were compared using Chi-Square, continuous
variables using t-tests or Mann-Whitney U (if applicable). Odds ratio’s were
derived from logistic regression models. Alpha was set to 0.05.
Results
210 consecutive pts were
screened and 169 pts were included (IMPT: n = 35, IMRT: n = 134). Median age
was 66 years, 53.3% were male, 40.8% had a squamous NSCLC and 41% of pts had a
WHO Performance Status (PS) =0. Median Gross Tumor volume (GTV) was 70.4 cm3.
No differences in age, gender, baseline PS, GTV and tumor histology were noted
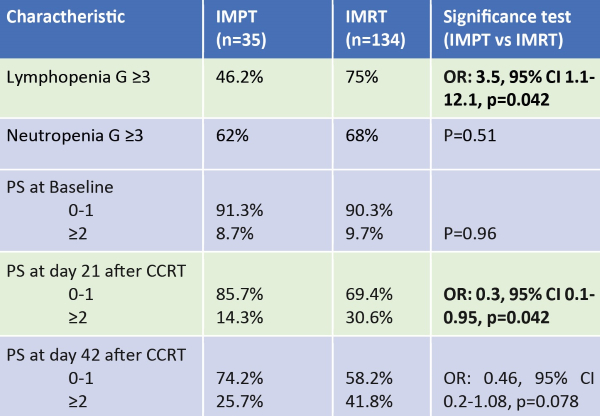
between IMPT and IMRT. 98.2% of the pts received a RT dose of 60-66Gy. 46.2% of
IMPT treated pts and 75% IMRT treated pts developed lymphopenia G ≥3 (Odds
Ratio [OR]: 3.5, 95% CI 1.1-12.1, p=0.042). Including age, comorbidities,
chemotherapy regimen, gender, disease stage (IIIA vs. IIIB/IIIC) and GTV in the
multivariate analysis, IMPT confirmed to be associated with less lymphopenia (OR:
0.07, 95% CI: 0.01-0.54, p=0.01). Neutropenia G ≥3 occurred in 62% and 68% in
IMPT and IMRT treated pts respectively (p=0.51). This was 31% and 29% respectively
for febrile neutropenia (p=0.74). Bone marrow RVs were associated with a higher
risk of lymphopenia G ≥3 (V4, V5, V10 and V20, with a significance level of
0.05, 0.034, 0.023, and 0.026 respectively). IMPT was also associated with a
lower rate of PS≥2 at day 21 (OR: 0.3, 95% CI 0.1-0.95, p=0.042) (table 1).
Conclusion
IMPT reduces the incidence
of lymphopenia G≥3 in
pts with stage III NSCLC treated with CCRT, due to lower bone marrow RVs. In
addition, IMPT led to a faster PS recovery after CCRT, thus potentially increasing
the number of patients eligible for adjuvant durvalumab.