Tamoxifen induces metabolic adaptations leading to radioresistance in breast cancer
Flavia Naumann,
The Netherlands
OC-0429
Abstract
Tamoxifen induces metabolic adaptations leading to radioresistance in breast cancer
Authors: Flavia Naumann1, Gosse Adema1, Fred Sweep2, Jan Bussink1, Paul Span1
1Radboud University Medical Center, Radiotherapy, Nijmegen, The Netherlands; 2Radboud University Medical Center, Laboratory Medicine, Nijmegen, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Tamoxifen
is a Selective Estrogen Receptor (ER) Modulator extensively used in the
adjuvant and first line treatment of ER positive breast cancer. Recently, we
reported that tamoxifen resistant breast cancer cells are cross-resistant to
irradiation, possibly hampering optimal treatment of breast cancer patients. Tamoxifen
has been shown to also exhibit ER-independent effects such as inhibition of
mitochondrial oxidative phosphorylation, which might explain subsequent
radioresistance. Here, we aim to investigate the mechanisms associated with tamoxifen
induced radioresistance, and whether this depends on ER status.
Material and Methods
To identify mechanisms underlying this
cross-resistance, we induced tamoxifen resistance in ER positive MCF7 and ER
negative MDA-MB-231 breast cancer cells by chronic treatment with increasing doses.
Several metabolic characteristics were assessed in wild type (WT) cells and
resistant (TAM) cells such as oxygen consumption and glycolysis, using the
Seahorse metabolic analyzer. Additionally, we measured real-time ROS production
in response to tamoxifen and H2O2 as well as toxicity of
these compounds to WT and TAM cells and assessed total antioxidant capacity.
Attempting to increase the sensitivity of TAM cells to ROS, NRF2, the main
activator for the antioxidant response element, was inhibited and the cells
sensitivity to several ROS as well as irradiation was examined.
Results
Clonogenic survival reveals that also MDA-MB-231
cells lacking an ER can become resistant to irradiation after chronic tamoxifen
treatment. We show that tamoxifen resistant cells exhibit a decreased oxygen
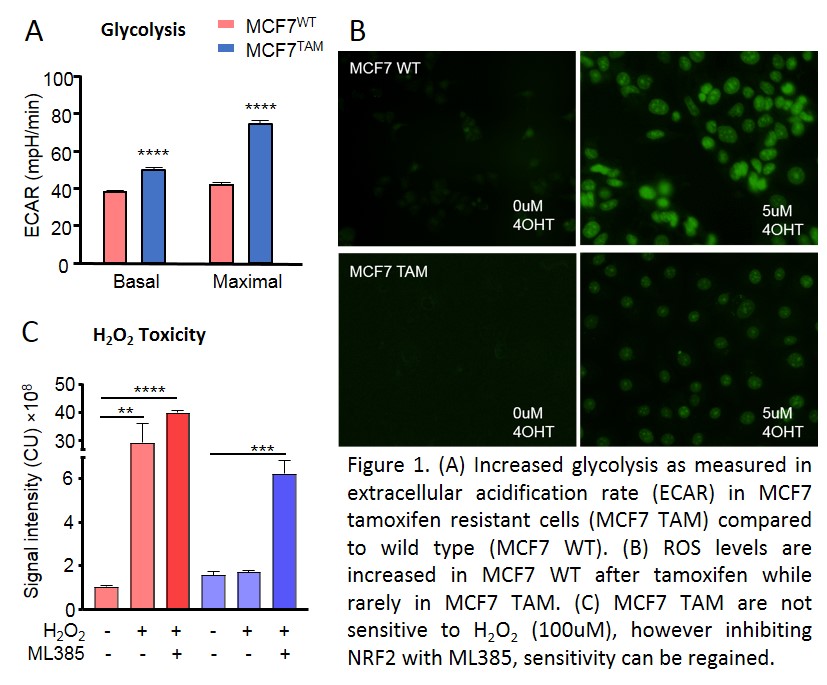
consumption rate and have developed a more glycolytic phenotype compared to
untreated wild type cells (Fig.1A), indicating mitochondrial dysfunction. Real
time measurement of ROS demonstrates significantly lower overall ROS levels in
resistant cells in response to tamoxifen (Fig.1), but also after H2O2
treatment. Additionally, we found treatment with H2O2 to
be less toxic on tamoxifen resistant cells, suggesting ROS protective
mechanisms as antioxidants to be more active in these cells. Indeed, we find
higher antioxidant levels in tamoxifen resistant cells likely protecting cells
from ROS induced DNA damage. By inhibiting NRF2, the activator of the antioxidant
response element, tamoxifen resistant cells reestablished their sensitivity to
ROS (Fig.1C).
Conclusion
These
data indicate two mechanisms of ER independent cellular adaptations underlying
tamoxifen induced radioresistance. We report an increased glycolytic capacity
in tamoxifen resistant cells, which is generally associated with
radioresistance. Additionally, increased metabolism of ROS and higher
expression of antioxidants serve to protect cells from DNA damage and death related
to irradiation induced ROS as we show that tamoxifen resistant cells regain
their sensitivity to ROS when NRF2 is inhibited which expectedly increases
sensitivity to irradiation.