Deep learning-based internal target volume adaption in SBRT
OC-0943
Abstract
Deep learning-based internal target volume adaption in SBRT
Authors: Lukas Wimmert1, Thilo Sentker2, Thore Dassow1, Frederic Madesta2, René Werner2, Tobias Gauer1
1University Medical Center Hamburg-Eppendorf, Radiotherapy and Radiation Oncology, Hamburg, Germany; 2University Medical Center Hamburg-Eppendorf, Computational Neuroscience, Hamburg, Germany
Show Affiliations
Hide Affiliations
Purpose or Objective
Internal target volume (ITV) definition is commonly carried out based on breathing-correlated 4DCT imaging. However, interfractional breathing variability may lead to sub-optimal ITV dimension over the course of SBRT despite applied motion management strategies. This work aims to optimize the ITV definition using deep learning-based prediction of the patient’s breathing amplitude range after the first dose fraction.
Material and Methods
The study includes 259 SBRT sessions of 234
patients with lung and liver lesions. For each session, 10-phase 4DCT data were
acquired for ITV definition. Patient breathing was recorded with Varian RPM
during 4DCT acquisition and during dose delivery in 5 fractions. The proposed
ITV optimization approach consists of two steps. (1) Deep learning modeling: a
convolutional neural network was applied using breathing curves of the 4DCT and
the first fraction (input data) to predict the amplitude range for the
following fractions. Corresponding ground truth is the optimal amplitude range.
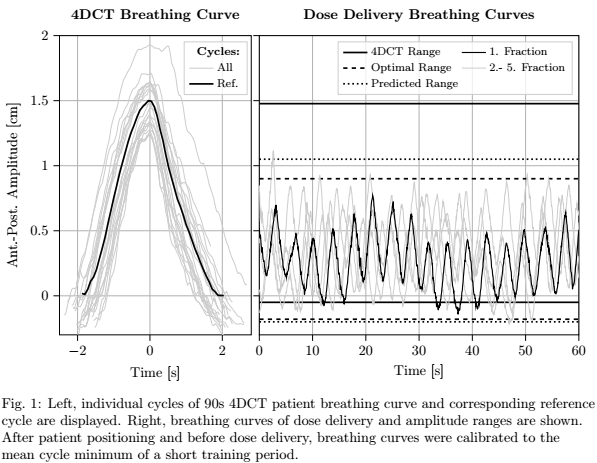
This range is defined retrospectively as coverage of all breathing amplitudes
acquired during dose delivery of fractions 2-5 except of extraordinary
irregularities (Fig. 1). Additionally, interfractional amplitude variability
was quantified by the Euclidean norm of the 4DCT amplitude range (given by
10-phase reference cycle) and the above optimal amplitude range (Fig. 1). For
network training and testing, SBRT sessions were split randomly in train
(n=191), validation (n=64) and test set (n=4, pre-selected). Model performance
is determined by prediction error (Euclidean norm of predicted and
retrospectively optimal amplitude range; Fig. 1). (2) ITV re-definition after
the first fraction: the initial ITV is adapted according to the patient’s
predicted breathing amplitude range by a 4DCT-based correspondence model that
correlates external breathing signal and internal tumor motion.
Results
(1) Model performance was 1.7 +/- 1.1 mm (mean
prediction error, validation set). Mean interfractional amplitude variability
was 3.4 +/- 2.4 mm. 4-fold cross validation obtained similar mean prediction
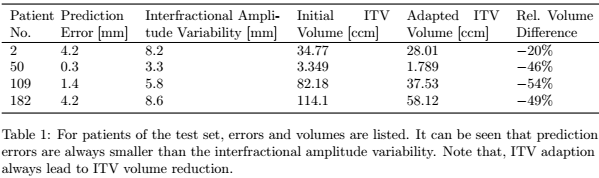
errors, indicating model reliability. (2) Results of ITV re-definition after
the first fraction are summarized in Table 1. The test set prediction error was
in the range of 0.3 to 4.2 mm and thus greater than for the validation set.
However, the test set interfractional amplitude variability was significantly
higher compared to the validation set. Despite reduction of initial ITV size,
the resulting adapted ITV would have achieved sufficient tumor motion coverage
during dose delivery when implemented in the clinics.
Conclusion
Our results support the assumption that
4DCT-based ITV definition may lead to unfavorable ITVs. However, this mainly
occurs in cases of adverse interplay of breathing-sensitive tumor motion and
interfractional variability of patient breathing. The proposed approach of ITV
re-definition reveals potential of significant ITV volume reduction and normal
tissue sparing.