Permanent alopecia in pediatric patients with Medulloblastoma treated by CSI: a threshold dose.
OC-0759
Abstract
Permanent alopecia in pediatric patients with Medulloblastoma treated by CSI: a threshold dose.
Authors: camilla satragno1, Antonio Verrico2, Salvina Barra3, Alfonso Ferrero1,7, Flavio Giannelli3, Francesca Cavagnetto4, Irene Schiavetti5, Maria Luisa Garrè2, Claudia Milanaccio6, Liliana Belgioia1,7, Renzo Giacinto Corvò1,3
1University of Genoa , Health Science Department (DISSAL) , Genova, Italy; 2IRCCS Istituto Giannina Gaslini , Paediatric Neuroncology, Genova, Italy; 3IRCCS San Martino Hospital, Radiation Oncology, Genova, Italy; 4IRCCS San Martino Hospital, Medical Physician, Genova, Italy; 5University of Genoa, Department of Health Sciences, Section Biostatistic, Genova, Italy; 6IRCCS Istituto Giannina Gaslini, Paediatric Neuroncology, Genova, Italy; 7IRCCS San Martino Hospital , Radiation Oncology, Genova, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
To identify a threshold dose constraint for permanent alopecia in patients with medulloblastoma (MB) treated with surgery followed by adjuvant chemotherapy (CT) and craniospinal irradiation (CSI).
Material and Methods
We retrospectively analyzed 42 patients (pts) with MB treated at our Institute, from 1999 to 2019. All pts had a minimum follow up of 12 months and underwent craniospinal irradiation (CSI) followed by a boost to whole posterior cranial fossa (W-PCF) and/or to the primary tumour site. Alopecia was assessed according to CTCAE v5.0 scale. Before 2010 pts were treated with 3D CRT technique. Since 2010 IMRT has been used for the PCF boost planning and from 2015 volumetric technique by helical Tomotherapy has become the standard treatment. In a subgroup of pts (N=27) the dose to the skin using Eclipse software (Varian Inc) was accurately assessed by scalp contouring. In this subgroup the skin was fully contoured across the scalp and manually defined as the volume from the outer surface of the skin to a depth of 3 mm up to a maximum thickness of 5mm.
Median dose (D), mean D, D98%, D2%, D50% and skin volume were estimated through DVH curves analysis of the whole skin and the W-PCF skin, in the CSI, boost area and sum plan. Any relationship between presence of alopecia (partial or complete) and other characteristics was investigated by an appropriate univariate and multivariate analysis. For each significant predictor variable, a receiver operating characteristic curve was drawn for calculating the Area Under the Curve and identifying the optimal discriminatory cut-off value for prediction of alopecia
Results
In this study we analyzed a cohort of MB survivors with a median follow up of 10 years.
Whole brain CSI doses ranged from 23.4 to 39.0 Gy. All 42 pts were boosted with either the entire PCF or the involved field of the tumour bed, with a total dose ranging from 54.0 to 69.0 Gy. High-dose and conventional chemotherapy were not significantly correlated with occurrence of permanent hair loss.
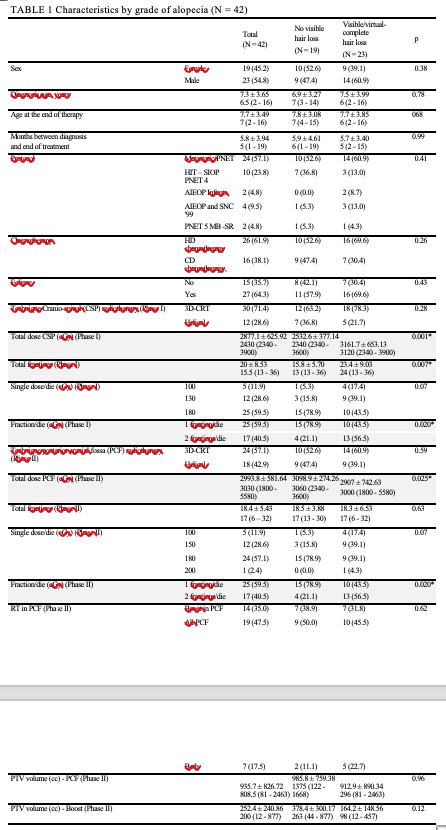
Table 1 shows characteristics of pts according to grade of toxicity (no hair loss vs partial/complete hair loss).
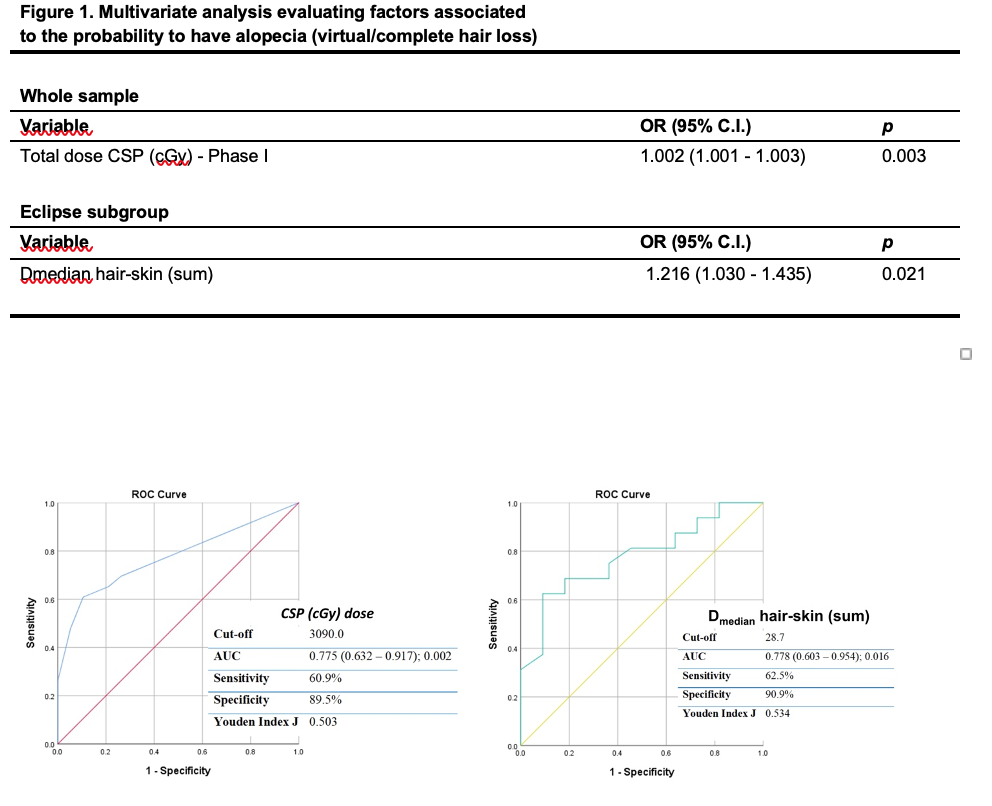
The best predictor for the occurrence of alopecia was the dose of CSI (OR: 1.002; 95%CI: 1.001-1.003; p=0.003), with an optimal discriminatory cut-off value of 30.9 Gy (Fig.1).
In Eclipse subgroup D2% and D50% were found to be significant only on univariate analysis.
The best predictor was D median hair skin (OR: 1.216; 95%CI: 1.030-1.435; p=0.021), with an optimal discriminatory cut-off value of 28.7 Gy (Fig. 1)
Conclusion
Our study has a very long median follow-up of about 10 years with a homogeneous population, pathology and radiation treatment.
Our analysis led to the identification of a precise threshold dose.
We need prospective confirmation of our results in larger cohorts that also take into account the assessment of quality of life.