Estimating risk of radiation toxicity for lymphoma patients using pre-chemotherapy PET-CT scans
Rebecca Shakir,
United Kingdom
OC-0292
Abstract
Estimating risk of radiation toxicity for lymphoma patients using pre-chemotherapy PET-CT scans
Authors: Rebecca Shakir1, Victoria Butterworth2, Johanna Ramroth1, David Cutter1, Georgios Ntentas3,2,1
1University of Oxford, Oxford Population Health, Oxford, United Kingdom; 2Guy's and St Thomas' NHS Foundation Trust, Department of Medical Physics, London, United Kingdom; 3King's College London, School of Biomedical Engineering and Imaging Sciences, London, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
Radiotherapy (RT) reduces the chance of lymphoma recurrence, but causes
late toxicity including heart disease and second cancers which can affect
quality and length of life. The risk of late toxicity is related to the
radiation dose received by organs from RT. A current limitation in estimating
risks of RT for an individual patient is that the dose to organs is not known until
after the decision to use RT has already been made and a RT plan produced.
The aim of this study was
to develop methods to predict the radiation dose organs would receive from RT,
and thereby the risks of RT, using the distribution of lymphoma on a patient's
pre-chemotherapy PET-CT scan. This would allow time for better-informed shared
decision making about the use of RT for lymphoma.
Material and Methods
Fifty patients treated for mediastinal Hodgkin or high-grade non-Hodgkin lymphoma between 2017-2020 with either full arc volumetric modulated arc therapy (VMAT, n=20) or butterfly VMAT (BVMAT, n=30) were included in the study. The distribution of lymphoma was documented from the pre-chemotherapy PET-CT scan, and the mean heart (MHD), lung (MLD) and breast (MBD) dose extracted from the RT plan. Separate multivariable linear regression models were built to predict MHD, MLD and MBD. Stepwise variable selection was used to select predictors for the final models. The process was repeated in 1000 bootstrap samples and the models chosen that most reduced variance. The modelled MHDs, MLDs and MBDs were used in combination with published radiation dose-response relationships to predict radiation-related relative risk (RR) from coronary heart disease (CHD), lung and breast cancer.
Results
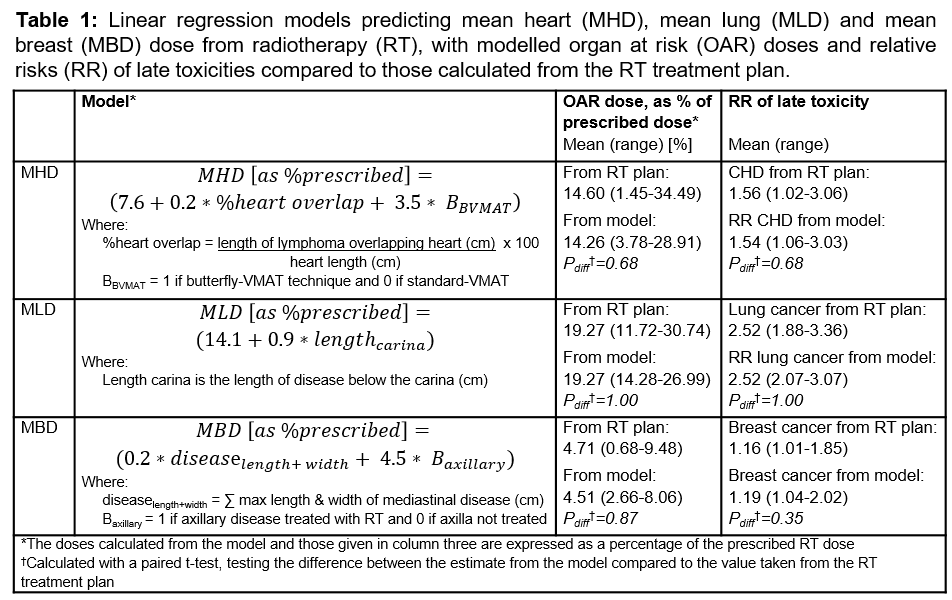
The models that best predicted MHD, MLD and MBD are shown in Table 1.
The variable that explained the majority of the variance in MHD was the
extent to which the lymphoma overlapped with the heart longitudinally,
expressed as a percentage of heart length. The final MHD model included this
and a RT technique variable. The length of disease below the carina best explained the variance in MLD. MBD was best explained using the
combination of the maximum length and width of mediastinal disease, together
with whether the axilla was included in the RT field.
Using these models, the
majority of patients had their MHD predicted to within 1.25Gy, MLD to within
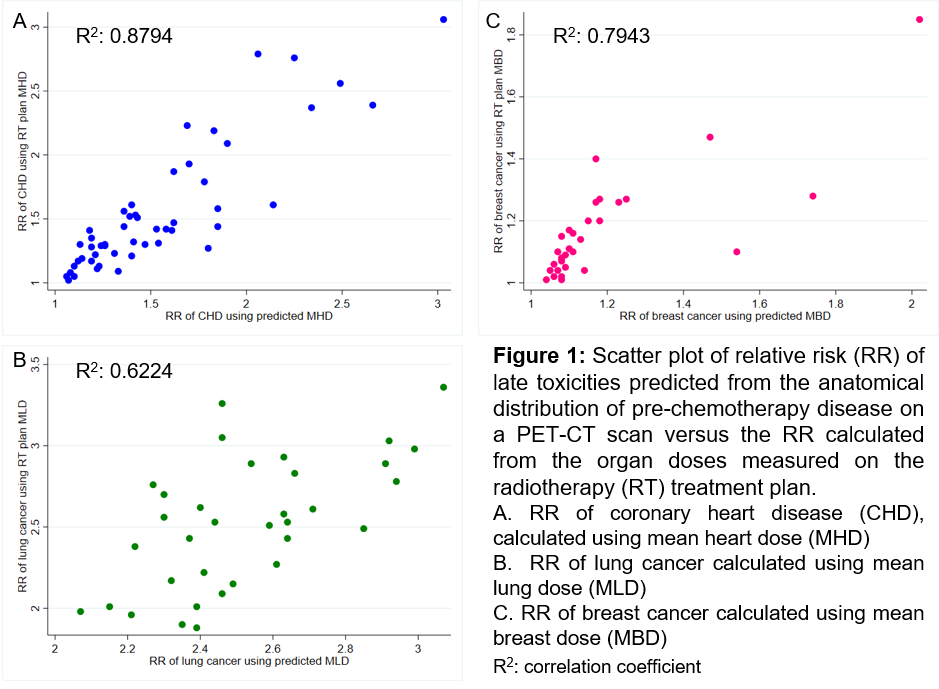
0.9Gy and MBD to within 0.5Gy. The individual patient RRs of CHD, lung and
breast cancer calculated using the predicted doses from the models were closely
correlated with the RRs calculated using doses from the RT plan (Figure 1). The
mean within-patient differences in RRs calculated using each of these methods
were not statistically significant (Table 1).
Conclusion
Patient-specific risks from consolidative RT can be estimated using pre-chemotherapy PET-CT scans. Such estimates can inform shared decision making about the use of RT to treat an individual with lymphoma. Work is ongoing to validate the models in a larger cohort, treated with a wider variety of RT techniques.