Personalized trade-off: elective nodal coverage vs. NTCP in head-and-neck cancer using automated MCO
Laura Patricia Kaplan,
Denmark
OC-0285
Abstract
Personalized trade-off: elective nodal coverage vs. NTCP in head-and-neck cancer using automated MCO
Authors: Laura Patricia Kaplan1,2,3,4, Linda Rossi4, Ben J. M. Heijmen4, Anne Ivalu Sander Holm1, Jesper Grau Eriksen5, Stine Sofia Korreman6,3,1
1Aarhus University Hospital, Department of Oncology, Aarhus, Denmark; 2Aarhus University, Departmenet of Clinical Medicine, Aarhus, Denmark; 3Aarhus University Hospital, Danish Centre for Particle Therapy, Aarhus, Denmark; 4Erasmus Medical Center, Department of Radiation Oncology, Rotterdam, The Netherlands; 5Aarhus University Hospital, Department of Experimental Clinical Oncology, Aarhus, Denmark; 6Aarhus University, Department of Clinical Medicine, Aarhus, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
The current
standard in curative radiotherapy (RT) for head-and-neck squamous cell
carcinoma (HNSCC) is to prioritize dose coverage highly for all patients, even
for the elective nodal CTV (CTVE). The risk of microscopic spread is
not uniform throughout CTVE, however. Some patients might benefit
from trading a slightly reduced dose in sub-volumes of CTVE where
risk of microscopic spread is lowest for lower organ-at-risk (OAR) doses. Our
aim was to develop an automated multi-criteria optimization (MCO) planning
workflow to systematically explore such patient-specific trade-offs between low-risk
CTVE coverage and normal tissue complication probability (NTCP).
Material and Methods
For 40
HNSCC patients, baseline VMAT plans (68/60/50 Gy SIB, PTV margin 5mm) were
retrospectively created following our clinical treatment protocol using an
in-house automated MCO software.
Sub-volumes
of CTVE/PTVE with lower risk of microscopic spread were
defined individually for each patient (termed trade-off CTVE/PTVE).
Trade-off CTVE was defined as the total CTVE minus lymph
levels containing a nodal metastasis (GTV + 1cm isotropic extension, see Fig.1).
The goal
for total PTVE near-minimum dose (D99%) was reduced from ≥47.5Gy to 45Gy and 42.5Gy in two trade-off plans (TP45/42.5).
Minimum dose to trade-off CTVE was constrained to 45Gy in both TPs
(47.5Gy in baseline plans). All other planning objectives and constraints (OARs
and remaining targets) were the same in all plans. Target dose reduction
relative to baseline plans was allowed only in trade-off CTVE/PTVE.
OAR doses, total
PTVE V47.5Gy, and NTCP for xerostomia and dysphagia (models
used in the DAHANCA35 study, based on doses to salivary glands or oral cavity
and swallowing muscles, respectively) were compared between baseline plans and
TPs. The location of voxels (interpolated to size 0.25x0.25x0.25 mm3)
with doses below 47.5 and 45Gy in trade-off PTVE was quantified by
distance to the volume’s outer contour.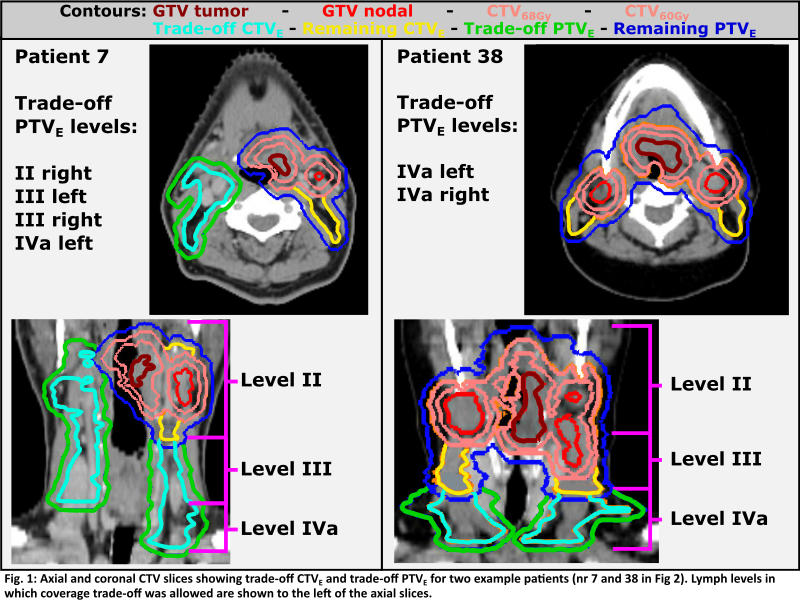
Results
Trade-off
PTVE volumes ranged from 6% to 73% of the total PTVE
volume (Fig.2, right). The trade-off possibilities thus varied between patients.
Reductions
in OAR mean dose, NTCP, and PTVE coverage are shown in Fig.2 and
below (median[min;max]).
| Contralateral
submandibular [Gy]
| Glottic
larynx [Gy]
| Esophagus
[Gy]
| PTVE V47.5Gy
[%]
|
TP45
| 1.5[0.1;6.1]
| 1.9[-0.9;7.0]
| 1.8[-0.1;12.7] | 2.4[1.2;5.2]
|
TP42.5
| 2.6[0.1;7.6]
| 2.9[-0.8;14.4]
| 2.7[0.4;13.8]
| 7.9[2.5;18.3]
|
Ninety-five
percent (95%) of trade-off PTVE
voxels below 47.5/45Gy in TPs were located within 10/4mm of the volume’s outer
edge.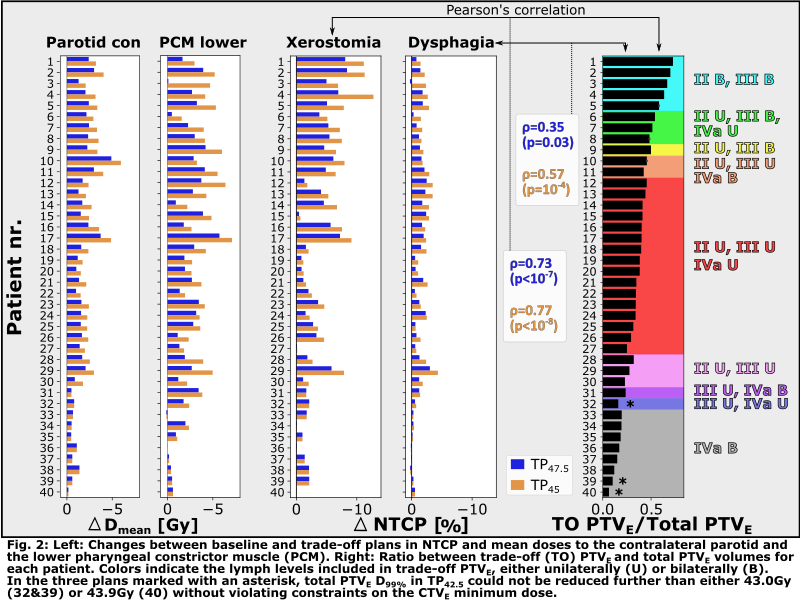
Conclusion
We have demonstrated
the possibility for substantial patient-specific reduction in OAR doses and
NTCP by limited and well-controlled coverage reduction in elective target sub-volumes
with low risk of microscopic disease spread. This workflow was made feasible by
using automated multi-criteria optimization.
The
proposed method may present a step towards novel planning strategies which systematically
include spatial variation in sub-clinical disease spread.