GEC-ESTRO Best Junior Presentation: Brachytherapy treatment verification using a moving phantom
Teun van Wagenberg,
The Netherlands
OC-0113
Abstract
Brachytherapy treatment verification using a moving phantom
Authors: Teun van Wagenberg1, Celine van Beveren1, Maaike Berbee1, Evert van Limbergen1, Frank Verhaegen1, Gabriel Paiva Fonseca1
1Maastricht University Medical Centre+, Department of Radiation Oncology (Maastro), Maastricht, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
High-dose rate (HDR) brachytherapy (BT) is a treatment modality with excellent clinical outcomes. However, in vivo dosimetry for BT is almost never performed due to the lack of treatment verification systems, and therefore information about the frequency and magnitude of errors that occur is lacking. Previous studies have shown that an imaging panel (IP) can be used to measure BT treatment parameters, including dwell times (<0.1s) and dwell coordinates (<1mm). So far measurements have always been performed using a static phantom, which is not entirely representative for a real clinical case. In this study, it will be explored whether similar results can be achieved using an in-house developed motion platform to move the phantom during measurements, mimicking patient movement.
Material and Methods
A treatment verification system comprised of a static IP and a 3D camera was used to track a custom 3D printed head phantom placed on a motion platform (Figure 1). Gamma rays from the radioactive source are caught by the IP and used to obtain information on source movement. The 3D camera is used to measure the external motion of the phantom. The measurements were performed while moving the phantom with a constant speed ranging from 3 mm/s up to 13 mm/s. The platform was used to simulate motion in 2 dimensions. The amplitude of the platform was set to 0.5 or 1 cm, having an uncertainty of 0.2 mm. A plan with several dwell positions of 1s was used to simulate a base of tongue treatment, with the needle being inserted in a cavity in the chin area of the head phantom.
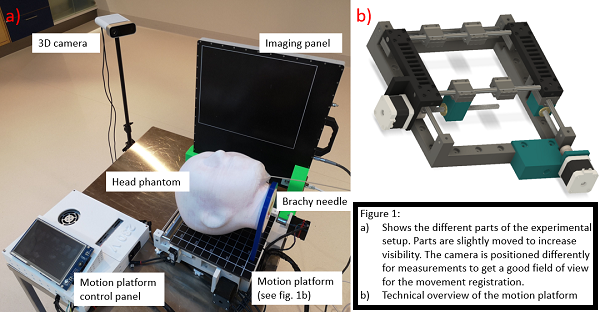
Results
The system identified all dwell positions, regardless of the movement speed and amplitude. The dwell times were determined within 0.1s, with results almost identical to those reported in a static phantom. The peaks of the absolute difference in the intensity plot are still prominent during motion, however, it can be noted that the values are increased in between two separate peaks when motion is applied (see figure 2). The height of the values in these valleys increases with the movement speed of the platform. Because of the high frame rate (10 fps) used to acquire images with the IP, it is possible to distinguish between patient and source motion, because the latter is much faster. Using the camera it was possible to find the amplitude applied to the head phantom with a submillimeter accuracy.
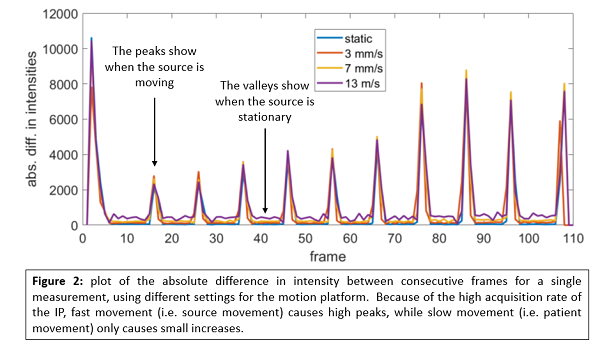
Conclusion
Previously published methods of dwell time determination can also be applied on a moving phantom, with a similar accuracy of <0.1s. Time resolved dosimetry using an IP and 3D camera allows for distinguishing between internal and external motion, providing information on dwell times even when the phantom is moving. Consequently, it is expected that this system could differentiate between external patient movement and source movement, and thus improve in vivo dosimetry for HDR BT.