RBE models of brainstem toxicity from proton therapy of paediatric ependymoma
Andreas Havsgård Handeland,
Norway
OC-0455
Abstract
RBE models of brainstem toxicity from proton therapy of paediatric ependymoma
Authors: Andreas Havsgård Handeland1, Daniel J. Indelicato2, Lars Fredrik Fjæra3, Kristian S. Ytre-Hauge3, Helge Egil S. Pettersen1, Yasmin Lassen-Ramshad4, Ludvig P. Muren5, Camilla H. Stokkevåg1,3
1Haukeland University Hospital, Department of Oncology and Medical Physics, Bergen, Norway; 2University of Florida, Department of Radiation Oncology, Jacksonville, USA; 3University of Bergen, Department of Physics and Technology, Bergen, Norway; 4Aarhus University Hospital, Danish Centre for Particle Therapy, Aarhus, Denmark; 5Aarhus University , Department of Medical Physics, Aarhus, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
Brainstem
necrosis is a rare, yet severe side-effect of paediatric radiotherapy. The
cause is likely multifactorial, with one possible contributor being uncertainty
in the relative biological effectiveness (RBE) of protons. A
constant RBE=1.1 is assumed clinically, but the RBE is known to vary with
linear energy transfer (LET), as well as radiosensitivity of tissue and dose fractionation.
Variable RBE-based predictive models are therefore needed for treatment plan
optimisation. The aim of this study was thus to investigate the effect of LET
and variable RBE in normal tissue complication probability (NTCP) modelling of
brainstem necrosis following paediatric ependymoma.
Results
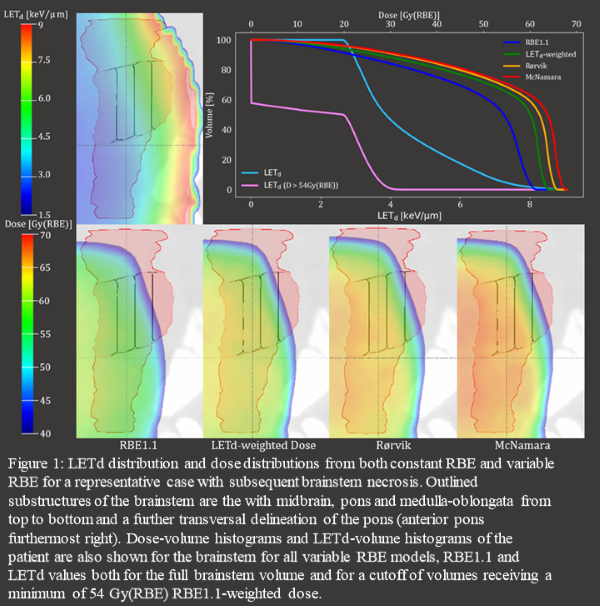
Variable RBE led to increased RBE-weighted dose
(figure 1). Most structures presented with 0.5–1.5keV/µm higher average L50%,
L10% (the LETd to the respective volume percentages) and Lmax (L0.1cc)
in cases compared to controls. These differences translated to ORs in the range
1.5–7 for full volumes and approaching 30 including dose thresholds. The difference in D50%,
D10% and Dmax
(D0.1cc) across cases and controls also increased from constant to variable RBE, with 0.5–1.5
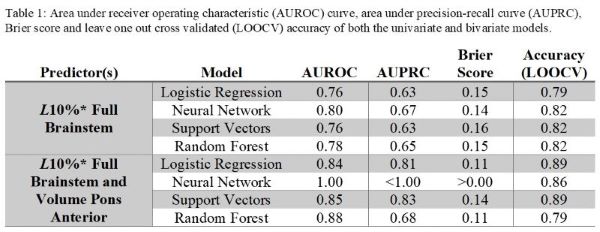
Gy(RBE) higher dose to cases with the McNamara model. The selected NTCP models were a univariate and bivariate
LR models achieving high performance scores (table 1).
The univariate model considered brainstem L10% (D>54 Gy(RBE) from RBE1.1),
while the bivariate model also included the anterior pons volume which was
negatively correlated with toxicity. The LR models had comparable performance
measures to most machine learning algorithms, excluding the bivariate neural
network with similar LOOCV accuracy only.
Conclusion
The most pronounced
risk was associated with increased LETd in volumes above 54Gy(RBE). The
univariate model incorporating brainstem L10% was the preferred model based on
simplicity and solid performance. The bivariate model, while more complex, is
strengthened by the inclusion of a parameter independent of dosimetry.