Combined radiomics and dosiomics predicts radiation pneumonitis : a model with external validation
Zhen Zhang,
The Netherlands
OC-0458
Abstract
Combined radiomics and dosiomics predicts radiation pneumonitis : a model with external validation
Authors: Zhen Zhang1,2, Leonard Wee1, Lujun Zhao2, Zhixiang Wang3, Andre Dekker3
1MAASTRO, Radiation Oncology, Maastricht, The Netherlands; 2Tianjin Medical University Cancer Institute and Hospital, Radiation Oncology, Tianjin, China; 3MAASTRO, Radiation Oncology , Maastricht, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Radiation pneumonitis (RP) is one of the common side effects of radiotherapy in the thoracic region. Radiomics and dosiomics quantifies information implicit within medical images and radiotherapy dose distributions. In this study we demonstrated the prognostic potential of radiomics, dosiomics and clinical features for RP prediction.
Material and Methods
Radiomics, dosiomics and clinical parameters were obtained on 314 retrospectively-collected and 35 prospectively-enrolled patients diagnosed with lung cancer between 2013 to 2019. A clinical risk score (C-score), radiomics risk score (R-score) and dosiomics risk score (D-score) were calculated based on logistic regression after feature selection. Seven models were built using different combinations of R-score, D-score, and C-score to evaluate their added prognostic power. Over-optimism was evaluated by bootstrap resampling from the training set, and the prospectively-collected cohort was used as the external test set. Model calibration and decision-curve characteristics of the best-performing models were evaluated. For ease of further evaluation, nomograms were constructed for selected models.
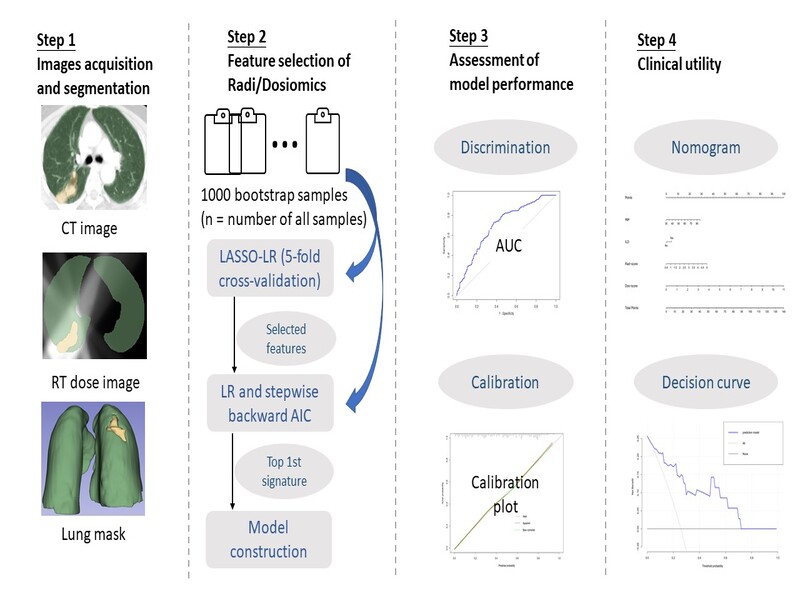
Figure
1.
Analysis flowchart. Step 1, The radiomics and dosiomics features of the lung
tissue region were extracted. Step 2, 1000 unique bootstrap
samples were taken from all samples, features were selected by correlation,
least absolute shrinkage (LASSO) embedded with logistic regression (LR) and
Akaike information criterion (AIC) for modeling. Step 3, The model performance
was evaluated using discrimination and calibration. Step 4, Clinical
applications were evaluated using nomogram and decision curves.
Results
A model built by integrating all of R-score, D-score and C-score had the best discriminative ability with area under the curves (AUCs) of 0.793,0.774 and 0.780 in the training set, bootstrapping set and external test set, respectively. The results of the calibration curve and decision curve analysis showed that the final model of the nomogram has potential for future clinical application.
Table 1 Discrimination ability of different models according to area under the curve (AUC) with the range provided between parentheses.
| Model | Train | Validation by bootstrapping | Testing |
| Radiomics | 0.676 (0.628-0.777) | 0.619 (0.541-0.639) | 0.636 (0.585-0.688) |
| Dosiomics | 0.728 (0.621-0.814) | 0.687 (0.639-0.704) | 0.630 (0.594-0.667) |
| Clinical | 0.664 (0.548-0.784)
| 0.654 (0.529-0.662) | 0.646 (0.625-0.676) |
| R-score+D-score | 0.735 (0.636-0.829) | 0.728 (0.692-0.731) | 0.679 (0.641-0.718) |
| R-score+C-score | 0.717 (0.594-0.816) | 0.701 (0.636-0.709) | 0.723 (0.692-0.756) |
| D-score+C-score | 0.770 (0.641-0.873) | 0.755 (0.724-0.771) | 0.770 (0.726-0.808) |
| R-score+D-score+C-score | 0.793 (0.667-0.891) | 0.774 (0.739-0.781) | 0.780 (0.744-0.821) |
Conclusion
Radiomics and dosiomics features have potential to assist with the prediction of RP, and the combination of radiomics, dosiomics and clinical parameters led to the best prognostic model in the present study.